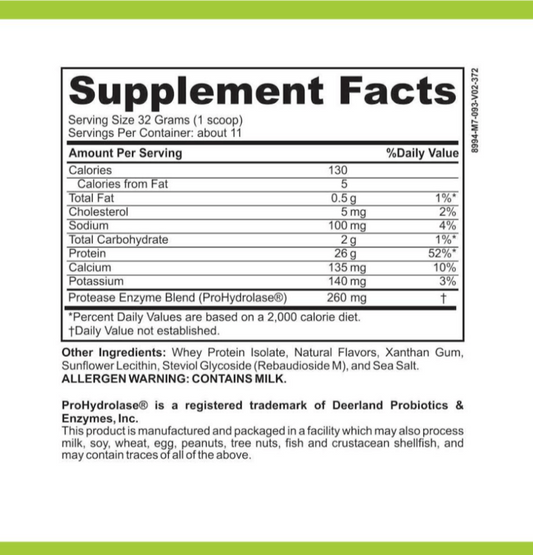
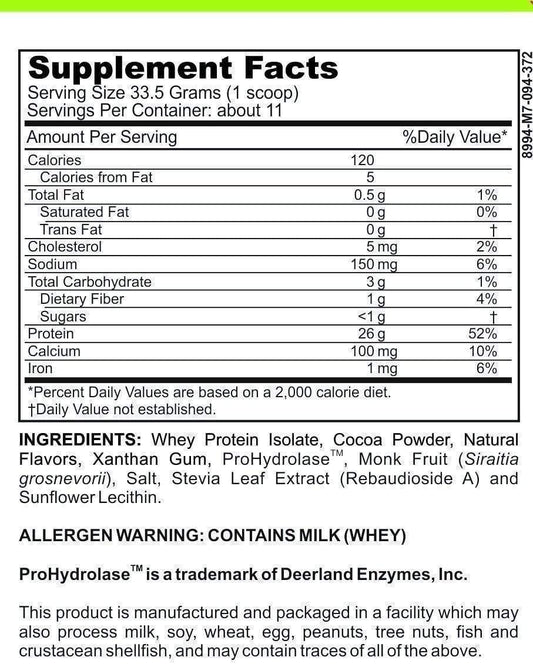
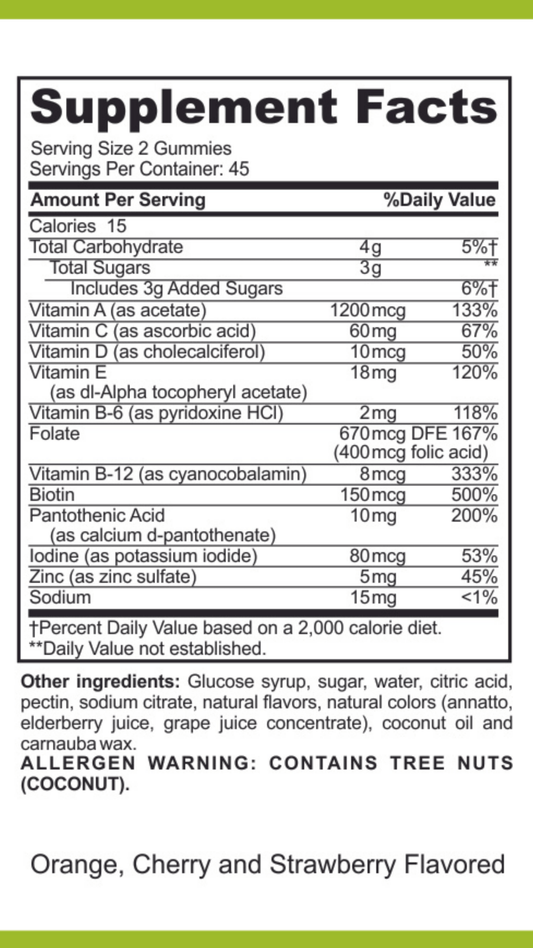
What Are Dietary Supplements Called In Canada
What Are Dietary Supplements Called In Canada
In Canada, dietary supplements are commonly referred to as natural health products (NHPs). These products are intended to supplement the diet and provide additional nutrients to support overall health and well-being. It is important to understand the terminology, regulatory framework, and safety considerations surrounding dietary supplements in Canada.
Understanding Dietary Supplements
Dietary supplements, or natural health products (NHPs), are defined as products that contain vitamins, minerals, herbal remedies, and other substances that are taken orally. These supplements are meant to enhance or supplement the diet and are available in various forms, including capsules, tablets, powders, and liquids.
When it comes to maintaining a healthy lifestyle, dietary supplements can be a valuable addition to one's routine. They are designed to provide essential nutrients that may be lacking in a person's diet, ensuring that the body receives the necessary vitamins and minerals for optimal functioning.
One of the key advantages of dietary supplements is their convenience. With the busy lives that many people lead, it can be challenging to consistently consume a well-balanced diet. Supplements offer a convenient alternative, providing a concentrated source of nutrients in a compact form. Whether it's a busy professional, a student juggling multiple responsibilities, or a parent on the go, dietary supplements can help bridge the nutritional gap.
Definition of Dietary Supplements
In Canada, dietary supplements are defined as natural health products (NHPs) under the Natural Health Products Regulations. These regulations specify that NHPs must be safe to use, have reasonable efficacy, and be of high quality.
Ensuring the safety and quality of dietary supplements is of utmost importance. Health regulatory bodies have established guidelines and regulations to protect consumers from potential harm. These regulations require manufacturers to adhere to strict quality control measures, including rigorous testing and certification processes. By complying with these regulations, manufacturers can provide consumers with the assurance that the dietary supplements they are purchasing are safe and effective.
Moreover, the definition of dietary supplements as natural health products highlights the emphasis on using natural ingredients. Many individuals prefer natural alternatives to conventional medicine, seeking products derived from plants, herbs, and other natural sources. This preference stems from the belief that natural ingredients are gentler on the body and may have fewer side effects compared to synthetic alternatives.
Importance of Dietary Supplements
Dietary supplements play an important role in supporting overall health and wellness. They can help address nutritional deficiencies, provide added support during specific life stages or health conditions, and optimize the body's functions.
One of the primary reasons people turn to dietary supplements is to address nutritional gaps in their diet. Despite efforts to eat a balanced diet, it can be challenging to obtain all the necessary nutrients solely from food. Factors such as busy schedules, limited food choices, and individual dietary restrictions can contribute to nutritional deficiencies. Dietary supplements can provide a convenient and reliable way to fill these gaps, ensuring that the body receives the essential nutrients it needs to function optimally.
Furthermore, dietary supplements can offer targeted support during specific life stages or health conditions. For example, pregnant women may benefit from prenatal supplements that provide essential vitamins and minerals for fetal development. Athletes and individuals engaging in intense physical activity may turn to sports supplements to enhance performance and aid in recovery. Additionally, individuals with specific health conditions, such as iron deficiency or weakened immune systems, may benefit from specialized supplements designed to address their unique needs.
Optimizing the body's functions is another area where dietary supplements can be beneficial. Certain nutrients play vital roles in supporting various bodily functions, such as immune system health, cognitive function, and bone strength. By ensuring an adequate intake of these nutrients through supplements, individuals can support their overall well-being and promote optimal physiological functioning.
Terminology of Dietary Supplements in Canada
When it comes to dietary supplements, Canada has specific terminology to describe and classify these products. Understanding the terminology is essential to make informed choices.
Canada takes the regulation of dietary supplements seriously, ensuring that consumers have access to safe and effective products. The terminology used in Canada encompasses a wide range of products that provide additional nutrients to support overall health and well-being.
Common Names for Dietary Supplements
Dietary supplements in Canada can be referred to as natural health products (NHPs), nutritional supplements, herbal remedies, or vitamins and minerals. These terms reflect the diverse range of products available to consumers, each with its own unique benefits and purposes.
Natural health products (NHPs) are derived from natural sources such as plants, animals, and microorganisms. They are intended to supplement the diet and promote health, including the prevention or treatment of disease. NHPs can include a variety of products such as vitamins, minerals, herbal remedies, homeopathic medicines, traditional medicines, probiotics, and other natural substances.
Nutritional supplements, on the other hand, focus specifically on providing essential nutrients that may be lacking in an individual's diet. These supplements can include vitamins, minerals, amino acids, fatty acids, and other substances that support overall nutrition and well-being.
Herbal remedies refer to dietary supplements that are derived from plants and are used for their medicinal properties. These remedies have been used for centuries in traditional medicine practices and are believed to provide various health benefits.
Vitamins and minerals are essential nutrients that the body needs in small amounts to function properly. They play a crucial role in maintaining overall health and are often taken as supplements to ensure adequate intake.
Legal Definitions and Terms
The legal definitions and terms related to dietary supplements in Canada are outlined in the Natural Health Products Regulations. These regulations provide a framework for the safe and effective use of dietary supplements, ensuring that products meet specific criteria for licensing, quality, and safety.
Product licensing is a crucial aspect of the regulations, ensuring that only approved products are available to consumers. Before a dietary supplement can be sold in Canada, it must undergo a rigorous review process to assess its safety, efficacy, and quality. This process involves evaluating the product's ingredients, manufacturing processes, and labeling information.
Product shelf life refers to the period during which a dietary supplement is expected to remain stable and maintain its quality. The regulations specify requirements for shelf life determination, ensuring that consumers can rely on the potency and effectiveness of the products they purchase.
Dosage forms and dosage units are also important considerations in the regulations. Different dietary supplements may come in various forms such as tablets, capsules, liquids, or powders. The regulations provide guidelines for the appropriate dosage forms and units to ensure accurate dosing and ease of use for consumers.
Labeling requirements are another crucial aspect of the regulations. The labeling of dietary supplements must provide clear and accurate information about the product, including its ingredients, recommended dosage, potential side effects, and any contraindications or warnings. This helps consumers make informed decisions and use the products safely.
By understanding the terminology and legal definitions surrounding dietary supplements in Canada, consumers can navigate the market with confidence, knowing that the products they choose meet strict regulatory standards and provide the intended benefits. It is always recommended to consult a healthcare professional before starting any new dietary supplement to ensure it is appropriate for individual needs and health conditions.
Regulatory Framework for Dietary Supplements in Canada
Canada has a regulatory framework in place to ensure the safety, quality, and efficacy of dietary supplements, also known as natural health products (NHPs).
Dietary supplements have become increasingly popular in Canada, with many individuals turning to these products to support their overall health and well-being. As a result, Health Canada has implemented a comprehensive regulatory framework to protect consumers and ensure that these products are safe and effective.
Health Canada's Role
Health Canada plays a crucial role in regulating dietary supplements in Canada. They are responsible for assessing product safety, issuing product licenses, and monitoring compliance with regulations.
Health Canada employs a team of experts who review scientific evidence and assess the safety and efficacy of dietary supplements before they can be legally sold in Canada. This rigorous evaluation process helps to ensure that only high-quality products that meet the necessary standards are available to consumers.
In addition to product assessment, Health Canada also provides guidance and support to manufacturers and distributors of dietary supplements. They offer resources and information on regulatory requirements, labeling guidelines, and good manufacturing practices to help companies navigate the complex regulatory landscape.
Compliance and Enforcement
Health Canada enforces strict regulations to ensure that dietary supplements meet the required safety and quality standards. This includes conducting inspections, responding to consumer complaints, and taking appropriate enforcement actions when necessary.
Health Canada's compliance and enforcement activities aim to identify and address any non-compliance issues in the dietary supplement industry. They conduct regular inspections of manufacturing facilities to ensure that proper quality control measures are in place and that products are being produced in accordance with the regulations.
In addition to inspections, Health Canada also relies on consumer feedback to identify potential safety concerns or non-compliant products. They encourage consumers to report any adverse reactions or quality issues they experience with dietary supplements, allowing them to take prompt action to protect public health.
When non-compliance is identified, Health Canada has a range of enforcement tools at their disposal. These can include product recalls, warning letters, fines, and even legal action in serious cases. By holding manufacturers and distributors accountable, Health Canada maintains the integrity of the dietary supplement market and safeguards the health of Canadian consumers.
In conclusion, Canada's regulatory framework for dietary supplements is designed to ensure that these products are safe, of high quality, and effective. Health Canada's role in assessing product safety, providing guidance to industry stakeholders, and enforcing compliance is crucial in maintaining consumer confidence and protecting public health.
Types of Dietary Supplements in Canada
Dietary supplements in Canada encompass a wide range of products. They can be classified into various categories based on their intended use and formulation.
Vitamins and Minerals
Vitamins and minerals are essential nutrients that our bodies need in specific amounts to maintain optimal health. They are available as individual supplements or in combinations to address specific nutrient deficiencies.
Herbal Supplements
Herbal supplements are derived from plants and are used for their medicinal properties. They can support overall health, address specific health conditions, and promote wellness.
Protein and Amino Acids
Protein and amino acid supplements are popular among individuals looking to support muscle growth, recovery, and overall fitness. These supplements provide the necessary building blocks for the body's tissues and can be beneficial for athletes or those with increased protein needs.
Safety and Efficacy of Dietary Supplements
The safety and efficacy of dietary supplements, also known as natural health products (NHPs), are paramount considerations for consumers.
Assessing the Safety of Dietary Supplements
Health Canada has stringent safety requirements for dietary supplements. Before a product can be legally sold in Canada, it must undergo a thorough safety assessment to ensure it does not pose any significant risks to consumers.
Understanding the Efficacy of Dietary Supplements
The efficacy of dietary supplements can vary depending on the specific product and the individual's unique needs. It is essential to consult with healthcare professionals and conduct thorough research to understand the potential benefits and limitations of a particular supplement.
Dietary supplements, or natural health products, play a significant role in supporting overall health and wellness in Canada. Understanding the terminology, regulatory framework, and safety considerations surrounding these supplements is essential for making informed choices. By staying informed and following appropriate guidelines, individuals can incorporate dietary supplements into their lifestyle in a safe and effective manner.