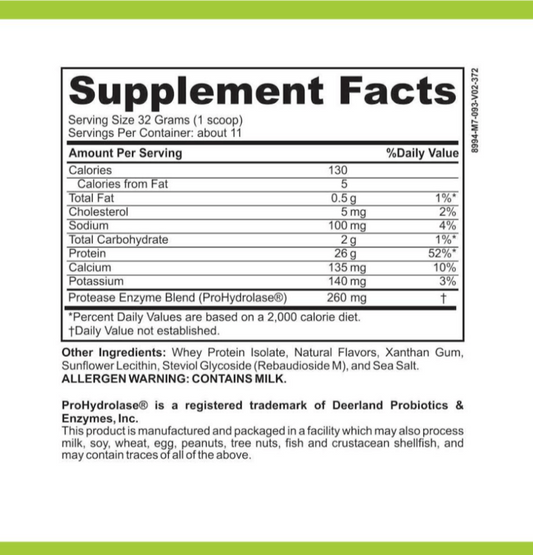
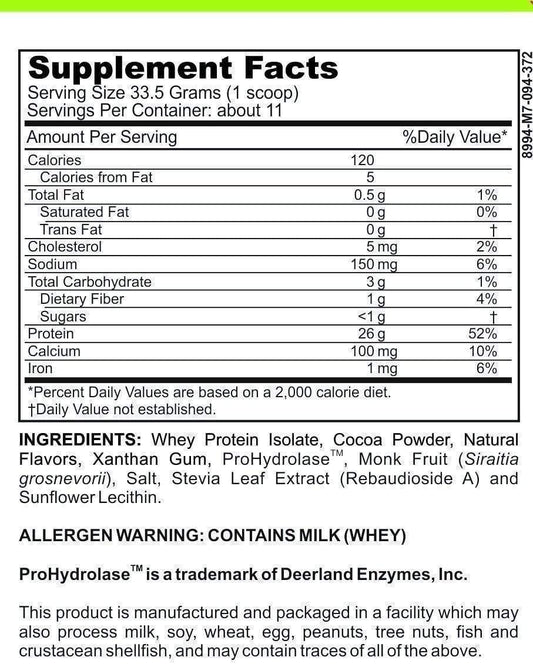
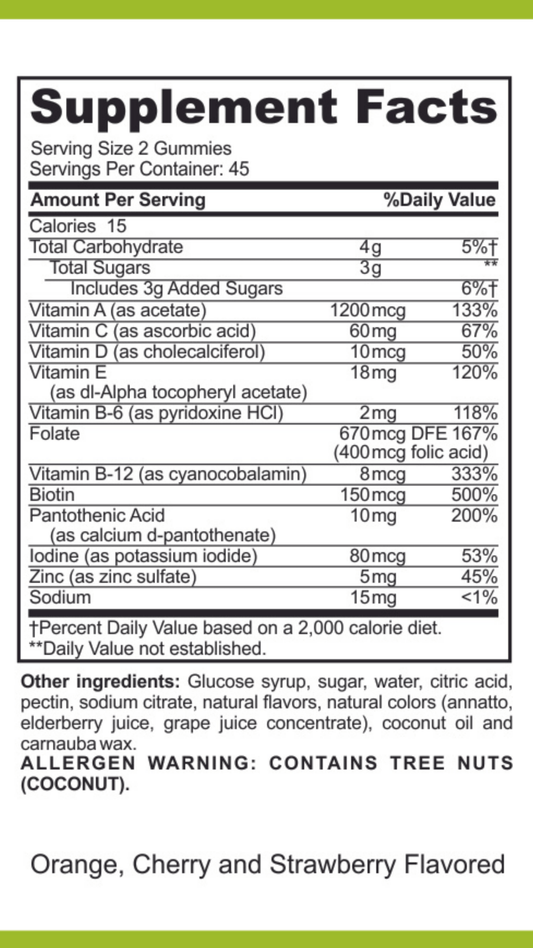
SIBO/IMO Glucose Breath Test By Commonwealth Diagnostics International, Inc. vs Helicobacter Pylori Antibodies
SIBO/IMO Glucose Breath Test By Commonwealth Diagnostics International, Inc. vs Helicobacter Pylori Antibodies
In the realm of gastrointestinal health, the SIBO/IMO Glucose Breath Test by Commonwealth Diagnostics International, Inc. and the detection of Helicobacter Pylori antibodies are two methods often employed to diagnose specific conditions. While both tests serve important diagnostic purposes, they differ significantly in terms of their scientific basis, procedures, and interpretation of results. This article aims to shed light on the intricacies of each method, compare their effectiveness in diagnosis, evaluate the pros and cons of each, and examine the comfort and convenience experienced by patients. Furthermore, this article will explore the future developments and innovations in these diagnostic tools, including advances in SIBO/IMO Glucose Breath Test technology and the progress in Helicobacter Pylori antibodies research.
Understanding SIBO/IMO Glucose Breath Test
The SIBO/IMO Glucose Breath Test is a non-invasive diagnostic tool used to identify the presence of Small Intestinal Bacterial Overgrowth (SIBO) and Intestinal Methane Overgrowth (IMO). This test is based on the scientific understanding that when there is an overgrowth of bacteria or other microorganisms in the small intestine, they metabolize the glucose or other substrates and produce specific gases, such as hydrogen and methane. By measuring these gases in the breath, clinicians can detect and diagnose SIBO/IMO.
The Science Behind SIBO/IMO Glucose Breath Test
The SIBO/IMO Glucose Breath Test relies on the principle that bacteria and certain microorganisms in the small intestine ferment carbohydrates, such as glucose, producing hydrogen and methane gases as byproducts. These gases are then absorbed into the bloodstream and exhaled through the breath. By collecting breath samples at specific intervals after ingesting a standardized glucose solution, the levels of hydrogen and methane can be measured and used as indicators of SIBO/IMO presence.
The process of bacterial fermentation in the small intestine is a complex biochemical reaction. The bacteria break down the carbohydrates into simpler compounds, releasing energy in the process. This energy is utilized by the bacteria for their own growth and survival. As a result of this metabolic activity, hydrogen and methane gases are produced as waste products. These gases are easily detectable in the breath, providing valuable insights into the microbial composition and activity within the small intestine.
Procedure of the SIBO/IMO Glucose Breath Test
The SIBO/IMO Glucose Breath Test involves several steps. Firstly, the patient is instructed to fast overnight before the test. This fasting period ensures that the small intestine is free from any recent food intake, allowing for accurate testing. Then, they consume a standardized glucose solution, usually containing a specific amount of glucose dissolved in water. The glucose solution serves as a substrate for the bacteria and microorganisms in the small intestine.
After ingesting the glucose solution, the patient is required to provide breath samples at specific intervals. These intervals are carefully chosen to capture the peak production and elimination of hydrogen and methane gases. Typically, breath samples are collected every 15 to 30 minutes for a duration of three hours. The collection of breath samples is done using a breath collection device, such as a breathalyzer or breath collection bag, which captures the exhaled gases.
Once the breath samples are collected, they are sent to a specialized laboratory for analysis. The laboratory utilizes sophisticated equipment to measure the levels of hydrogen and methane in each breath sample. The analysis involves comparing the gas concentrations in the breath samples to establish baseline values and identify any abnormalities that may indicate the presence of SIBO/IMO.
Interpreting the Results of SIBO/IMO Glucose Breath Test
The interpretation of SIBO/IMO Glucose Breath Test results involves analyzing the levels of hydrogen and methane measured in the breath samples. Elevated levels of these gases indicate the presence of bacterial overgrowth in the small intestine. However, it is important to note that the interpretation of the results is not solely based on the presence or absence of these gases. The specific patterns and concentrations of hydrogen and methane can provide further insights into the types of bacteria present and their metabolic activities.
Medical professionals, utilizing established diagnostic criteria, cross-reference the measured values with established thresholds to determine if SIBO/IMO is present and to what extent. The interpretation of the results requires expertise and knowledge of the normal ranges and variations in gas production. Additionally, the clinical presentation and symptoms of the patient are also taken into consideration to make an accurate diagnosis.
In conclusion, the SIBO/IMO Glucose Breath Test is a valuable tool in the diagnosis of Small Intestinal Bacterial Overgrowth and Intestinal Methane Overgrowth. By measuring the levels of hydrogen and methane gases in the breath, clinicians can gain insights into the microbial composition and activity in the small intestine, helping to guide appropriate treatment strategies for patients with SIBO/IMO.
An Overview of Commonwealth Diagnostics International, Inc.
Commonwealth Diagnostics International, Inc. is a reputable healthcare company specializing in gastrointestinal diagnostics. With a mission to improve patient care through accurate and reliable testing, Commonwealth Diagnostics has made significant contributions to the field of SIBO/IMO diagnostics. They offer comprehensive breath testing solutions and work closely with healthcare providers to detect and diagnose gastrointestinal disorders effectively.
History and Background of Commonwealth Diagnostics International, Inc.
Founded in [insert year], Commonwealth Diagnostics International, Inc. has been at the forefront of gastrointestinal diagnostics for several decades. The company's commitment to continuous research and innovation has enabled the development of cutting-edge diagnostic tools, including the SIBO/IMO Glucose Breath Test. Commonwealth Diagnostics has established itself as a trusted partner in the medical community, providing accurate and timely results to assist in the diagnosis and treatment of gastrointestinal conditions.
Role of Commonwealth Diagnostics in SIBO/IMO Glucose Breath Test
Commonwealth Diagnostics plays a vital role in the implementation and interpretation of the SIBO/IMO Glucose Breath Test. They partner with healthcare providers to ensure reliable testing protocols and accurate analysis of breath samples. Commonwealth Diagnostics also offers educational resources and support to healthcare professionals, ensuring they have the necessary expertise to effectively diagnose and manage SIBO/IMO cases.
Helicobacter Pylori Antibodies Explained
Helicobacter Pylori is a type of bacteria that can infect the stomach lining, leading to various gastrointestinal conditions, including peptic ulcers and gastritis. Detection of Helicobacter Pylori antibodies is a commonly used method to diagnose this bacterial infection. Understanding the role of antibodies in Helicobacter Pylori detection is crucial in evaluating the effectiveness of this diagnostic approach.
Understanding Helicobacter Pylori Infection
Helicobacter Pylori infection occurs when the bacteria enter the stomach and attach themselves to the gastric mucosa. This infection triggers an immune response within the body, leading to the production of specific antibodies targeted against the Helicobacter Pylori bacteria. Testing for the presence of these antibodies can indicate whether the individual has been exposed to, or is currently infected with, Helicobacter Pylori.
The Role of Antibodies in Helicobacter Pylori Detection
When Helicobacter Pylori infects the stomach lining, the immune system produces antibodies, specifically Immunoglobulin G (IgG), Immunoglobulin A (IgA), and Immunoglobulin M (IgM), as a response to the presence of the bacteria. These antibodies circulate in the bloodstream and can be detected using laboratory tests. The detection of Helicobacter Pylori antibodies in serum or plasma provides evidence of exposure to, or current infection with, the bacteria.
Comparing SIBO/IMO Glucose Breath Test and Helicobacter Pylori Antibodies
Both the SIBO/IMO Glucose Breath Test and the detection of Helicobacter Pylori antibodies serve crucial diagnostic purposes, albeit for different conditions. By comparing these diagnostic methods, we can gain insights into their effectiveness in diagnosing each respective condition, evaluate the advantages and disadvantages of each approach, and understand the comfort and convenience experienced by patients when undergoing these tests.
Effectiveness in Diagnosis
The effectiveness of a diagnostic method depends on its ability to accurately detect and diagnose the condition of interest. The SIBO/IMO Glucose Breath Test has been shown to be a reliable and sensitive tool in identifying small intestinal bacterial overgrowth and intestinal methane overgrowth. The detection of elevated levels of hydrogen and methane gases in the breath provides quantitative evidence of bacterial overgrowth, aiding in accurate diagnosis. On the other hand, the detection of Helicobacter Pylori antibodies has been widely used in diagnosing Helicobacter Pylori infection. However, it is important to note that antibody tests alone do not provide definitive evidence of an active infection, as the presence of antibodies can indicate past exposure or current infection.
Pros and Cons of Each Method
Both the SIBO/IMO Glucose Breath Test and Helicobacter Pylori antibody tests have their respective advantages and limitations. The SIBO/IMO Glucose Breath Test offers a non-invasive, quick, and convenient diagnostic method that can provide real-time results. This test allows for the detection of specific breath gases, providing quantitative data to aid in treatment decisions. However, it requires specific sample collection devices and specialized equipment for analysis. In contrast, the detection of Helicobacter Pylori antibodies is relatively simple, cost-effective, and widely available. It can be performed using blood samples, making it easily accessible in various healthcare settings. However, antibody tests do not differentiate between active or past infections, potentially leading to false-positive or false-negative results.
Patient Experience: Comfort and Convenience
When it comes to patient experience, the SIBO/IMO Glucose Breath Test is generally well-tolerated, as it involves consuming a glucose solution and providing breath samples over a defined testing duration. The test is non-invasive, eliminating the need for invasive procedures such as endoscopy. On the other hand, the detection of Helicobacter Pylori antibodies typically involves a blood test, which may cause discomfort for some individuals. However, blood tests are generally considered safe and pose minimal risk to patients.
Future Developments and Innovations
Advancements in diagnostic technology and research continue to drive the development of more accurate and efficient tools for diagnosing gastrointestinal conditions. Both the SIBO/IMO Glucose Breath Test and Helicobacter Pylori antibody detection are areas of active research and innovation.
Advances in SIBO/IMO Glucose Breath Test Technology
Ongoing research aims to enhance the SIBO/IMO Glucose Breath Test by improving the accuracy and precision of breath sample collection and analysis. Advances in sensor technology, breath collection devices, and data analysis algorithms contribute to the development of more sensitive and reliable diagnostic tools for detecting and monitoring bacterial overgrowth in the small intestine.
Progress in Helicobacter Pylori Antibodies Research
Researchers are continually working to improve the reliability and specificity of Helicobacter Pylori antibody tests. The development of novel detection techniques and examination of different antibody types, including IgG, IgM, and IgA, contribute to the refinement of these tests. Furthermore, efforts are underway to develop rapid point-of-care antibody tests that provide timely results, enabling immediate treatment decisions.
In conclusion, the SIBO/IMO Glucose Breath Test by Commonwealth Diagnostics International, Inc. and the detection of Helicobacter Pylori antibodies serve as valuable diagnostic tools in gastrointestinal health. While the SIBO/IMO Glucose Breath Test offers a non-invasive, quantitative approach to detect small intestinal bacterial overgrowth and intestinal methane overgrowth, the Helicobacter Pylori antibody test allows for the diagnosis of Helicobacter Pylori infection. Understanding the scientific basis, procedures, and interpretation of results for each method is essential for healthcare providers and patients alike. As research and innovation continue, these diagnostic tools are poised to become even more effective in diagnosing gastrointestinal conditions, ultimately improving patient care and outcomes.