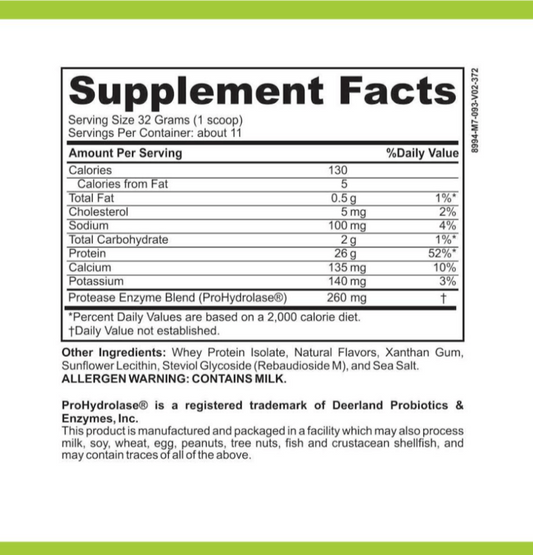
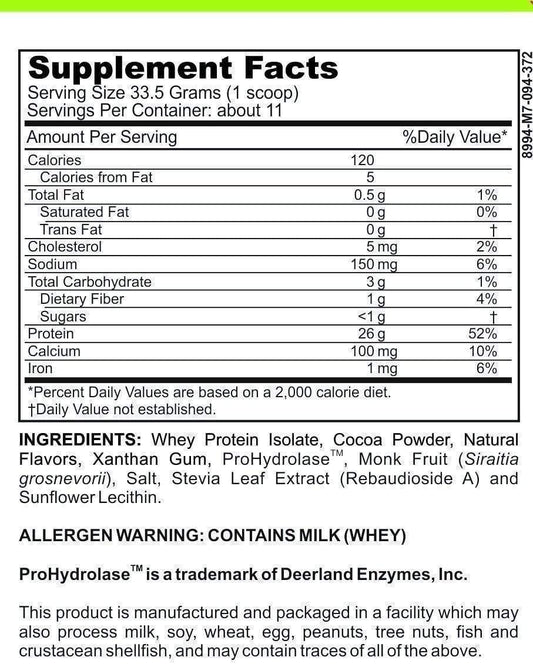
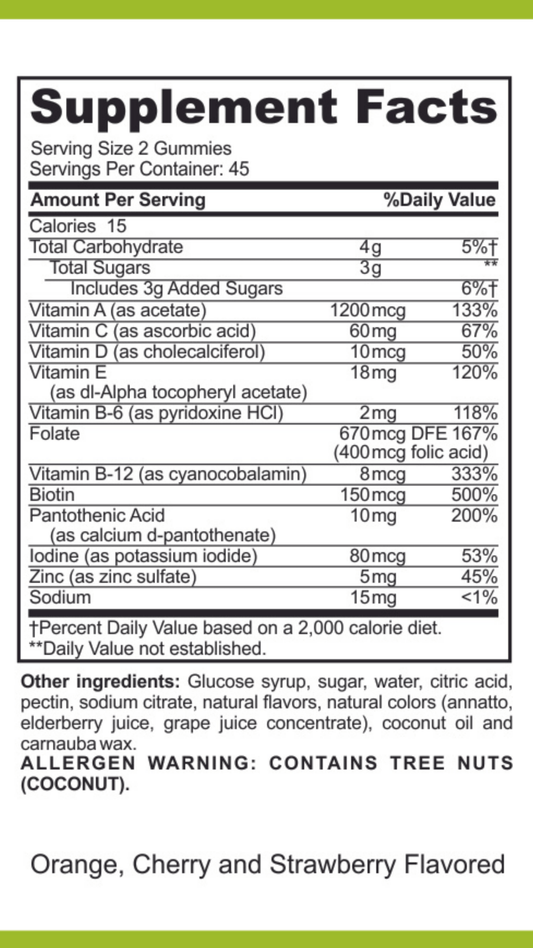
SIBO/IMO Glucose Breath Test By Commonwealth Diagnostics International, Inc. vs E. Coli Shiga Toxins
SIBO/IMO Glucose Breath Test By Commonwealth Diagnostics International, Inc. vs E. Coli Shiga Toxins
The SIBO/IMO Glucose Breath Test conducted by Commonwealth Diagnostics International, Inc. and the presence of E. Coli Shiga Toxins are two important factors that impact human health. In this article, we will delve deeper into understanding the SIBO/IMO Glucose Breath Test, the role of Commonwealth Diagnostics International, Inc. in this test, and the impact of E. Coli Shiga Toxins on human health. We will also compare the diagnostic accuracy of these tests and review case studies and clinical trials. Lastly, we will explore future perspectives and draw conclusions based on the information presented.
Understanding SIBO/IMO Glucose Breath Test
The SIBO/IMO Glucose Breath Test is a diagnostic tool used to detect Small Intestinal Bacterial Overgrowth (SIBO) and Intestinal Methane Overgrowth (IMO). It involves measuring levels of hydrogen and methane gases in a patient's breath following the consumption of a glucose solution.
What is SIBO/IMO Glucose Breath Test?
The SIBO/IMO Glucose Breath Test is a non-invasive procedure that provides valuable insights into the presence and severity of SIBO and IMO. By measuring the gases produced by bacteria in the small intestine, healthcare professionals can accurately diagnose and manage these conditions.
When a patient undergoes the SIBO/IMO Glucose Breath Test, they are given a glucose solution to drink. This solution serves as a substrate for the bacteria in the small intestine. As the bacteria break down the glucose, they produce hydrogen and methane gases as byproducts.
How Does the SIBO/IMO Glucose Breath Test Work?
The SIBO/IMO Glucose Breath Test works by analyzing the fermentation of glucose by bacteria in the small intestine. When bacteria break down glucose, they produce hydrogen and methane gases, which are then absorbed into the bloodstream and exhaled through the breath. By measuring the levels of these gases, healthcare professionals can determine the presence and severity of SIBO or IMO.
After consuming the glucose solution, the patient will be instructed to breathe into a collection device at specific intervals. The device captures their breath and measures the concentration of hydrogen and methane gases. These measurements are then analyzed to determine if the patient has SIBO, IMO, or both.
The SIBO/IMO Glucose Breath Test is a safe and reliable method for diagnosing and managing SIBO and IMO. It offers a non-invasive alternative to invasive procedures such as endoscopy or colonoscopy, making it more comfortable for patients.
The Role of Commonwealth Diagnostics International, Inc. in SIBO/IMO Glucose Breath Test
Commonwealth Diagnostics International, Inc. plays a crucial role in the development and provision of the SIBO/IMO Glucose Breath Test. They are dedicated to advancing the field of gastrointestinal diagnostics and providing healthcare professionals with accurate and reliable testing methodologies.
As a leader in breath testing, Commonwealth Diagnostics International, Inc. collaborates with healthcare providers to develop innovative testing protocols and technologies. Their expertise ensures that the SIBO/IMO Glucose Breath Test delivers accurate results, enabling healthcare professionals to make informed decisions about patient care.
Furthermore, Commonwealth Diagnostics International, Inc. is committed to ongoing research and development to improve the accuracy and efficiency of the SIBO/IMO Glucose Breath Test. They strive to stay at the forefront of diagnostic advancements, ensuring that healthcare providers have access to the latest tools and knowledge in the field of gastrointestinal diagnostics.
In conclusion, the SIBO/IMO Glucose Breath Test is a valuable diagnostic tool for detecting and managing Small Intestinal Bacterial Overgrowth (SIBO) and Intestinal Methane Overgrowth (IMO). With the support of Commonwealth Diagnostics International, Inc., healthcare professionals can confidently diagnose and treat patients with these conditions, leading to improved patient outcomes and quality of life.
E. Coli Shiga Toxins: An Overview
E. Coli Shiga Toxins are a group of harmful substances produced by certain strains of Escherichia coli (E. Coli) bacteria. These toxins can cause severe gastrointestinal symptoms, such as diarrhea, stomach cramps, and even kidney damage in some cases.
E. Coli Shiga Toxins are classified into two main types: Stx1 and Stx2. These toxins are produced by bacteria that have acquired specific genes known as stx genes. The presence of these genes allows the bacteria to produce the toxins and release them into the surrounding environment.
When a person ingests food or water contaminated with E. Coli bacteria that produce Shiga Toxins, the toxins can enter the body and cause damage to the lining of the intestines. This damage can lead to the release of inflammatory substances and the disruption of normal intestinal function.
The Impact of E. Coli Shiga Toxins on Human Health
The presence of E. Coli Shiga Toxins in the body can lead to serious health complications. Infection with these toxins can result in a condition known as Hemolytic Uremic Syndrome (HUS), which can cause kidney failure and other potentially life-threatening complications.
HUS is characterized by the destruction of red blood cells, which can lead to anemia and a decrease in the ability of the blood to carry oxygen. The damaged red blood cells can also clog the small blood vessels in the kidneys, leading to kidney damage and a decrease in kidney function.
In addition to kidney damage, E. Coli Shiga Toxins can also affect other organs in the body. The toxins can cause damage to the lining of blood vessels, leading to the formation of blood clots. These blood clots can then travel to different parts of the body, causing damage to organs such as the brain, heart, and lungs.
It is important to note that not all strains of E. Coli bacteria produce Shiga Toxins. However, when outbreaks of E. Coli infection occur, it is crucial to identify the specific strain involved and determine if it is producing Shiga Toxins. This information is essential for proper diagnosis and treatment of affected individuals.
In conclusion, E. Coli Shiga Toxins are harmful substances produced by certain strains of E. Coli bacteria. These toxins can cause severe gastrointestinal symptoms and have the potential to lead to serious health complications, such as Hemolytic Uremic Syndrome. Understanding the impact of these toxins on human health is crucial for effective prevention and treatment strategies.
Comparing SIBO/IMO Glucose Breath Test and E. Coli Shiga Toxins
Similarities and Differences
While both the SIBO/IMO Glucose Breath Test and E. Coli Shiga Toxins impact human health, they differ in terms of their mechanisms and diagnostic approaches. The SIBO/IMO Glucose Breath Test aims to identify bacterial overgrowth in the small intestine, while E. Coli Shiga Toxins specifically target the gastrointestinal system and can cause severe complications.
Diagnostic Accuracy: SIBO/IMO Glucose Breath Test vs E. Coli Shiga Toxins
Both tests have shown high diagnostic accuracy in detecting the respective conditions they are designed for. The SIBO/IMO Glucose Breath Test has been proven effective in identifying bacterial overgrowth, while specific laboratory tests are used to detect the presence of E. Coli Shiga Toxins in stool samples.
Let's delve deeper into the SIBO/IMO Glucose Breath Test. This test is based on the principle that certain bacteria in the small intestine can ferment glucose, producing hydrogen gas as a byproduct. By measuring the levels of hydrogen in a patient's breath after ingesting a glucose solution, healthcare professionals can determine if there is an overgrowth of bacteria in the small intestine. This overgrowth, known as Small Intestinal Bacterial Overgrowth (SIBO), can lead to symptoms such as bloating, abdominal pain, and diarrhea.
On the other hand, E. Coli Shiga Toxins are produced by certain strains of Escherichia coli (E. Coli) bacteria. These toxins are responsible for causing severe gastrointestinal infections, with symptoms ranging from mild diarrhea to bloody diarrhea and even life-threatening complications like hemolytic uremic syndrome (HUS). The presence of E. Coli Shiga Toxins in the gastrointestinal system can be detected through laboratory tests that analyze stool samples. These tests are crucial in diagnosing and managing E. Coli infections, especially in outbreaks or cases of suspected contamination.
While the SIBO/IMO Glucose Breath Test focuses on identifying bacterial overgrowth in the small intestine, the detection of E. Coli Shiga Toxins targets a specific strain of bacteria and its harmful toxins. This difference in scope highlights the unique mechanisms and diagnostic approaches of these two tests.
It is important to note that both the SIBO/IMO Glucose Breath Test and the detection of E. Coli Shiga Toxins have demonstrated high diagnostic accuracy. The SIBO/IMO Glucose Breath Test has been extensively studied and validated as a reliable method for identifying bacterial overgrowth in the small intestine. Similarly, laboratory tests for E. Coli Shiga Toxins have been developed and refined to ensure accurate detection of these toxins in stool samples. These tests play a crucial role in diagnosing and managing the respective conditions, allowing healthcare professionals to provide appropriate treatment and care for patients.
In conclusion, while the SIBO/IMO Glucose Breath Test and the detection of E. Coli Shiga Toxins share the common goal of impacting human health, they differ in their mechanisms and diagnostic approaches. The SIBO/IMO Glucose Breath Test focuses on identifying bacterial overgrowth in the small intestine, while the detection of E. Coli Shiga Toxins specifically targets the presence of these harmful toxins in the gastrointestinal system. Both tests have shown high diagnostic accuracy, enabling healthcare professionals to effectively diagnose and manage the conditions they are designed for.
Case Studies and Clinical Trials
Case Study: SIBO/IMO Glucose Breath Test
In a recent case study conducted by Commonwealth Diagnostics International, Inc., a patient with suspected SIBO underwent the SIBO/IMO Glucose Breath Test. The test results confirmed the presence of SIBO, allowing healthcare professionals to tailor an appropriate treatment plan and monitor the patient's progress over time.
Case Study: E. Coli Shiga Toxins
In another case study, patients who presented with symptoms of E. Coli infection were tested for the presence of Shiga toxins. The analysis of stool samples revealed the presence of the toxins, confirming the diagnosis and enabling the healthcare team to provide timely and targeted treatment.
Future Perspectives and Conclusions
The Future of SIBO/IMO Glucose Breath Testing
As research and technology continue to advance, the SIBO/IMO Glucose Breath Test may undergo further refinements, leading to even higher levels of accuracy and efficiency. This will ensure better patient outcomes and improved diagnostic capabilities in identifying SIBO and IMO.
The Future of E. Coli Shiga Toxins Research
Ongoing research is critical in understanding the mechanism of action and the long-term effects of E. Coli Shiga Toxins. Advancements in this field may lead to the development of targeted therapies and preventive measures to mitigate the impact of these toxins on human health.
Final Thoughts and Conclusions
In conclusion, the SIBO/IMO Glucose Breath Test and E. Coli Shiga Toxins are two important factors to consider when evaluating gastrointestinal health. The SIBO/IMO Glucose Breath Test, offered by Commonwealth Diagnostics International, Inc., provides an effective diagnostic tool for identifying bacterial overgrowth in the small intestine. On the other hand, E. Coli Shiga Toxins can cause serious health complications, particularly in cases of Hemolytic Uremic Syndrome. By understanding these tests and their impact, healthcare professionals can better diagnose and manage these conditions, leading to improved patient outcomes and increased overall well-being.