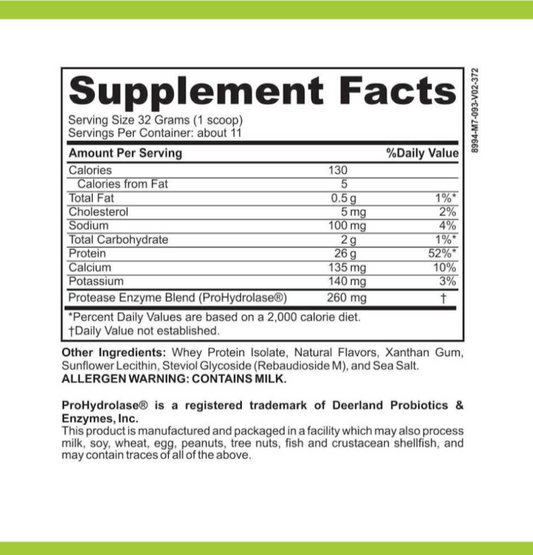
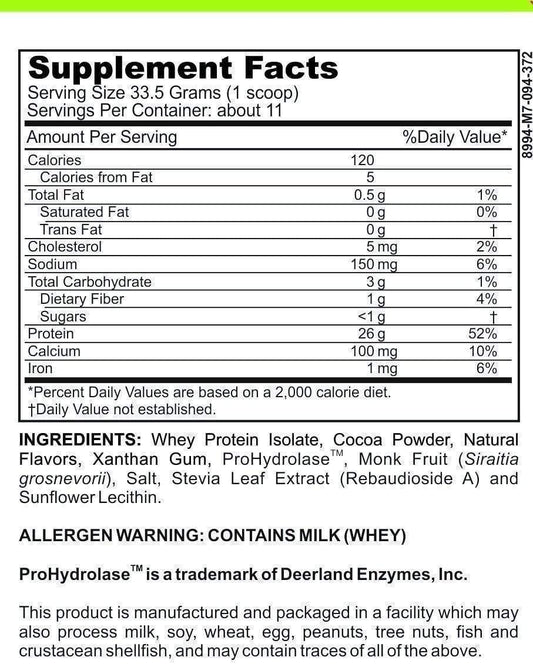
Is Calcium Oxide High In Histamine
Is Calcium Oxide High In Histamine
Calcium oxide, also known as quicklime or burnt lime, is a commonly used chemical compound with various industrial applications. In recent years, there has been some speculation about the potential histamine content in calcium oxide and its implications for health and safety. In this article, we will explore the composition and role of calcium oxide, the biological function of histamine, and the relationship between calcium oxide and histamine. We will also discuss the implications of high histamine levels and safety measures when handling calcium oxide. Finally, we will summarize the current knowledge and highlight potential areas for future investigation.
Understanding Calcium Oxide
Calcium oxide, also known as quicklime or burnt lime, is an inorganic compound that is synthesized through the thermal decomposition of calcium carbonate. This process, known as calcination, involves heating calcium carbonate at high temperatures to release carbon dioxide and produce calcium oxide. It is a white crystalline solid at room temperature and is extensively used in numerous industrial processes.
Calcium oxide, with its chemical formula CaO, is composed of one calcium atom (Ca) and one oxygen atom (O). This compound is highly reactive and readily reacts with water to form calcium hydroxide, a process known as slaking. The reaction between calcium oxide and water is exothermic, releasing a significant amount of heat. This property makes calcium oxide useful in various applications.
The Chemical Composition of Calcium Oxide
Chemically, calcium oxide is an alkaline earth oxide. It belongs to a group of compounds known as metal oxides, which are formed when metals react with oxygen. Calcium oxide is classified as a basic oxide due to its ability to react with acids to form salts and water. It has a molar mass of 56.08 grams per mole and a density of 3.34 grams per cubic centimeter.
Calcium oxide has a cubic crystal structure, with calcium ions occupying the corners of the unit cell and oxygen ions located at the center of each face. This arrangement gives calcium oxide its characteristic properties, such as its high melting point of 2,572 degrees Celsius and its low solubility in water.
The Role of Calcium Oxide in Various Industries
Calcium oxide has widespread industrial applications due to its unique properties and versatility. One of its primary uses is in the production of cement. When calcium oxide reacts with water, it forms calcium hydroxide, which acts as a binding agent in the cement mixture. This process, known as hydration, is essential for the hardening and setting of concrete.
In addition to its role in cement production, calcium oxide is used as a flux agent in metallurgical processes. It helps remove impurities from metal ores during smelting, allowing for the production of high-quality metals. Calcium oxide can also be used as a desiccant, or drying agent, to remove moisture from gases and liquids. Its ability to absorb water makes it valuable in industries such as natural gas processing and chemical manufacturing.
Furthermore, calcium oxide finds applications in the manufacturing of paper, glass, and ceramics. In the paper industry, it is used as a filler to improve the paper's opacity and smoothness. In glass production, calcium oxide acts as a stabilizer, enhancing the strength and durability of the glass. It is also a crucial ingredient in the production of ceramics, providing stability and enhancing the properties of the final product.
Overall, calcium oxide plays a vital role in various industries, contributing to the development of infrastructure, the production of metals, and the creation of everyday materials. Its unique properties and versatility make it a valuable compound with a wide range of applications.
Histamine: A Brief Overview
Histamine is a naturally occurring molecule in the human body and plays a crucial role in various physiological functions. It is a biogenic amine that acts as a neurotransmitter and is involved in regulating immune responses, gastric acid secretion, and allergic reactions.
The Biological Function of Histamine
Histamine is primarily known for its role in allergic reactions. When the body encounters an allergen, such as pollen or pet dander, mast cells release histamine as part of the immune response. Histamine then binds to specific receptors on nearby cells, triggering a cascade of events that result in the characteristic symptoms of allergies, such as itching, redness, and swelling.
However, histamine's functions extend beyond allergies. It is also involved in regulating stomach acid secretion, which plays a crucial role in the digestion process. In the stomach, histamine acts on specific receptors called H2 receptors, located on the surface of parietal cells. When histamine binds to these receptors, it stimulates the production of gastric acid, aiding in the breakdown of food and facilitating nutrient absorption.
Furthermore, histamine acts as a neurotransmitter in the central nervous system, where it participates in various physiological processes. It plays a role in wakefulness and sleep regulation, as well as in the control of appetite and body temperature. Histamine receptors are widely distributed throughout the brain, and their activation or inhibition can have profound effects on these functions.
Common Sources of Histamine
Histamine can be found in various foods, which can be a concern for individuals who are sensitive to its effects. Aged cheese, such as blue cheese and Parmesan, is known to contain higher levels of histamine. Fermented products, such as sauerkraut, soy sauce, and yogurt, also have varying amounts of histamine. Additionally, certain types of fish, such as tuna, mackerel, and sardines, are known to contain high levels of histamine, especially if they are not stored properly.
It is important to note that histamine can also be generated in the body by the enzyme histidine decarboxylase. This enzyme is responsible for converting the amino acid histidine into histamine. When an allergic response or inflammation occurs, histidine decarboxylase is activated, leading to an increase in histamine production. This can further contribute to the symptoms experienced during an allergic reaction or inflammatory process.
In conclusion, histamine is a multifunctional molecule that plays a crucial role in various physiological processes. While it is primarily known for its involvement in allergic reactions, histamine also regulates stomach acid secretion and acts as a neurotransmitter in the central nervous system. Understanding the sources and functions of histamine can help individuals manage their allergies and make informed dietary choices.
The Relationship Between Calcium Oxide and Histamine
Calcium oxide, also known as quicklime, has long been recognized for its industrial uses, such as in cement production and water treatment. However, an intriguing area of research that has received relatively little attention is the potential presence of histamine in calcium oxide.
Histamine, a biogenic amine, is commonly associated with allergic reactions and is known to play a role in various physiological processes in the human body. While calcium oxide has been extensively studied for its industrial applications, its potential histamine content has only recently become a subject of scientific inquiry.
The Potential for Histamine in Calcium Oxide
Preliminary studies have suggested that calcium oxide may contain trace amounts of histamine. However, the exact extent of this presence and its implications are still not fully understood. Researchers have employed various analytical techniques to detect histamine in calcium oxide samples, but the results have been inconsistent.
Some studies have detected minimal levels of histamine in calcium oxide, while others have found no trace of it at all. These conflicting findings raise important questions about the factors that influence histamine content in calcium oxide and the potential sources of histamine contamination.
It is worth noting that the significance of these findings in terms of human exposure and potential health effects remains unclear. Further research is needed to determine whether the histamine levels detected in calcium oxide are of concern and whether they could pose any risks to human health.
Scientific Research on Calcium Oxide and Histamine
To establish a definitive connection between calcium oxide and histamine, more rigorous scientific investigations are necessary. Researchers should employ robust analytical methods and larger sample sizes to obtain more accurate and reliable results.
Moreover, it would be valuable to conduct studies that consider the potential release of histamine during the industrial processes involving calcium oxide. Understanding the conditions under which histamine may be released from calcium oxide could provide valuable insight into occupational health and safety measures.
Additionally, further research is needed to explore the potential mechanisms by which histamine could be present in calcium oxide. This could involve investigating the sources of histamine contamination, such as the raw materials used in the production of calcium oxide or the storage conditions of the compound.
Overall, while preliminary studies have hinted at the potential presence of histamine in calcium oxide, more comprehensive research is required to fully elucidate this relationship. By expanding our knowledge in this area, we can better understand the implications of calcium oxide use and ensure the safety of workers and consumers alike.
Implications for Health and Safety
While the exact relationship between calcium oxide and histamine warrants further examination, it is essential to consider potential health effects and safety measures associated with both compounds.
Calcium oxide, also known as quicklime or burnt lime, is a white, caustic, alkaline crystalline solid that is widely used in various industries. It is primarily used in the production of cement, steel, and chemicals. However, it is important to note that calcium oxide can pose health risks if not handled properly.
Possible Health Effects of High Histamine Levels
Excessive histamine levels in the body can lead to allergic reactions, including itching, hives, and respiratory difficulties. Histamine is a chemical compound that plays a crucial role in the immune system, but when its levels become imbalanced, it can cause adverse effects.
In severe cases, histamine toxicity can occur, leading to a condition known as histamine intolerance. This condition is characterized by an inability to properly break down and eliminate histamine from the body, resulting in a range of symptoms such as headaches, digestive issues, and skin problems.
It is important for individuals who suspect they may have histamine intolerance to consult with a healthcare professional for proper diagnosis and management.
Safety Measures When Handling Calcium Oxide
When working with calcium oxide, it is crucial to follow proper safety protocols to prevent potential harm. Calcium oxide is highly reactive with water, releasing a large amount of heat and producing calcium hydroxide, also known as slaked lime.
Protective gear, such as gloves, goggles, and appropriate ventilation, should be used to minimize exposure to calcium oxide dust or fumes. Direct contact with calcium oxide can cause severe skin irritation and burns, while inhalation of its dust or fumes can irritate the respiratory system.
Adequate training and supervision are also essential to ensure safe handling and disposal of calcium oxide. Workers should be educated on the potential hazards associated with calcium oxide and trained on proper handling techniques to minimize the risk of accidents or injuries.
In addition, it is important to store calcium oxide in a dry and well-ventilated area, away from incompatible substances such as acids or water. Proper labeling and signage should be in place to alert individuals of the presence of calcium oxide and its associated hazards.
In the event of accidental exposure or ingestion of calcium oxide, immediate medical attention should be sought. It is crucial to provide healthcare professionals with detailed information about the substance to ensure appropriate treatment.
By implementing these safety measures and promoting awareness of the potential health effects, individuals can minimize the risks associated with both histamine imbalance and the handling of calcium oxide.
Conclusions and Future Research Directions
In conclusion, the potential histamine content in calcium oxide is an intriguing area of research that requires further investigation. While preliminary studies have indicated the presence of trace amounts of histamine, the significance and implications of these findings remain uncertain. Additional research should focus on exploring potential histamine release during industrial processes and establishing a definitive connection between calcium oxide and histamine.
Summarizing the Current Knowledge
Currently, the available scientific literature provides limited evidence to support the claim that calcium oxide is high in histamine. The existing studies have produced inconclusive results, and no established link between calcium oxide and histamine has been established.
Potential Areas for Future Investigation
Future research should aim to address these gaps in knowledge and consider various factors, including reliable analytical methods, industrial practices, and human exposure assessments. Investigating the potential release of histamine during calcium oxide-related processes would be instrumental in enhancing our understanding of this relationship.
As our understanding of calcium oxide and histamine continues to evolve, it is important to approach this topic with scientific rigor and remain open to new findings that could shape our understanding of the potential interaction between these two compounds.