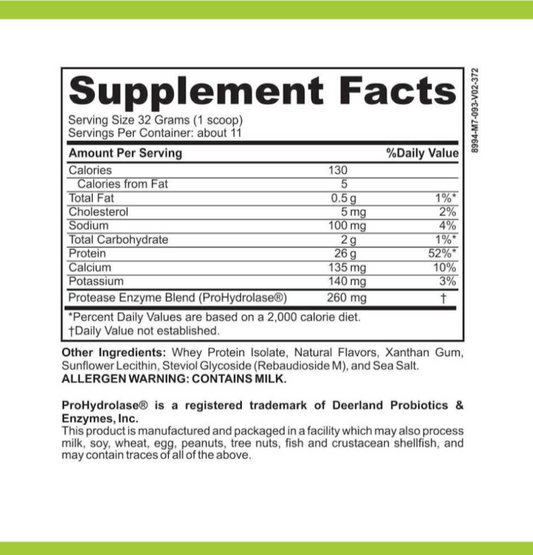
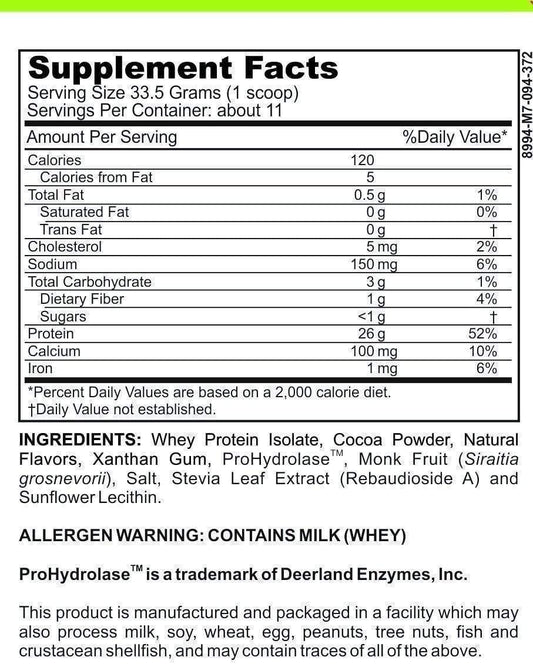
Fodmate Enzymes: Understanding Their Role in Metabolic Processes
Fodmate Enzymes: Understanding Their Role in Metabolic Processes
Enzymes are the unsung heroes of our metabolic processes, working tirelessly to facilitate the chemical reactions that keep us alive and functioning. Among these remarkable biological catalysts, Fodmate enzymes have emerged as particularly significant players in various metabolic pathways. Despite their importance, many people outside specialized scientific fields remain unaware of these enzymes and their critical functions in both human health and industrial applications.
Fodmate enzymes belong to a specialized class of biocatalysts that facilitate the breakdown and conversion of specific carbohydrate compounds, particularly those found in certain dietary components that can cause digestive distress in sensitive individuals. Their discovery has revolutionized our understanding of digestive processes and opened new avenues for treating metabolic disorders.
The Biochemistry of Fodmate Enzymes
At their core, Fodmate enzymes are protein molecules with highly specific three-dimensional structures that enable them to bind to particular substrates. Like all enzymes, they work by lowering the activation energy required for chemical reactions, thereby dramatically increasing reaction rates without being consumed in the process. What makes Fodmate enzymes special is their specificity for certain carbohydrate bonds that are otherwise difficult for the human digestive system to break down.
The name "Fodmate" derives from their primary function: facilitating the metabolism of fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs). These compounds are found in many foods and can cause significant digestive issues for people with irritable bowel syndrome (IBS) and other gastrointestinal disorders.
Structural Characteristics
Fodmate enzymes possess a distinctive protein structure that includes a catalytic site specifically shaped to accommodate FODMAP substrates. X-ray crystallography studies have revealed that these enzymes typically feature a deep binding pocket lined with amino acid residues that create an ideal microenvironment for the reaction to occur. This pocket contains both hydrophobic and hydrophilic regions that help position the substrate correctly for catalysis.
Most Fodmate enzymes belong to the hydrolase class, meaning they catalyze the addition of water to break chemical bonds. Their active sites often contain acidic and basic amino acid residues that participate directly in the catalytic mechanism, frequently involving a nucleophilic attack on the glycosidic bonds that hold sugar molecules together.
Catalytic Mechanisms
The catalytic process of Fodmate enzymes typically follows a two-step mechanism. First, the enzyme binds to the substrate, causing a slight conformational change that positions the reactive groups optimally. This binding step is highly specific and is often described using the "lock and key" or "induced fit" models of enzyme action. Second, the actual chemical reaction occurs, breaking the targeted bond and releasing the products.
What's particularly fascinating about Fodmate enzymes is their remarkable efficiency. A single Fodmate enzyme molecule can process thousands of substrate molecules per second, making them incredibly effective even at low concentrations. This efficiency is crucial for their biological function, especially in environments where the target substrates may be present in relatively small amounts.
Types of Fodmate Enzymes and Their Functions
The Fodmate enzyme family encompasses several distinct enzymes, each specialized for different substrates. Understanding these variations helps explain their diverse roles in metabolic processes and their potential applications in medicine and biotechnology.
Alpha-Galactosidase
Alpha-galactosidase is perhaps the most well-known member of the Fodmate enzyme family. This enzyme specifically targets the alpha-galactosidic bonds found in complex carbohydrates like raffinose and stachyose, which are abundant in legumes, certain vegetables, and whole grains. Humans naturally produce small amounts of alpha-galactosidase, but often not enough to completely digest these compounds, leading to fermentation by gut bacteria and resulting gas production.
Supplemental alpha-galactosidase has gained popularity as a digestive aid, commonly marketed under brand names like Beano. These supplements help break down otherwise indigestible carbohydrates before they reach the large intestine, reducing gas, bloating, and discomfort after consuming foods like beans, lentils, and cruciferous vegetables.
Lactase
Lactase is another crucial Fodmate enzyme that hydrolyzes lactose, the primary sugar in milk and dairy products, into its component monosaccharides: glucose and galactose. Lactose intolerance, affecting approximately 65% of the global population, results from insufficient lactase production. This condition leads to undigested lactose passing into the colon, where bacteria ferment it, causing symptoms like abdominal pain, bloating, and diarrhea.
Lactase supplements have become essential for many individuals with lactose intolerance, allowing them to enjoy dairy products without discomfort. Additionally, the food industry uses lactase extensively to produce lactose-free dairy products, expanding dietary options for lactose-intolerant consumers.
Fructan Hydrolases
Fructan hydrolases break down fructans, which are chains of fructose molecules found in foods like wheat, onions, and garlic. These enzymes are particularly relevant for individuals following low-FODMAP diets to manage IBS symptoms. Unlike lactase and alpha-galactosidase, human bodies don't naturally produce enzymes capable of breaking down fructans in the small intestine.
Research into supplemental fructan hydrolases is ongoing, with promising results for reducing symptoms in IBS patients. These enzymes could potentially allow sensitive individuals to consume fructan-containing foods with reduced digestive distress, significantly improving their quality of life and dietary flexibility.
Metabolic Pathways Involving Fodmate Enzymes
Fodmate enzymes participate in several critical metabolic pathways, influencing not only digestion but also energy production, cellular signaling, and even immune function. Understanding these pathways provides insight into why these enzymes are so important for overall health.
Carbohydrate Metabolism
The primary role of Fodmate enzymes lies in carbohydrate metabolism, particularly in breaking down complex carbohydrates that would otherwise remain undigested. In the small intestine, these enzymes work alongside other digestive enzymes to convert dietary carbohydrates into absorbable monosaccharides. This process is essential for extracting energy from food and maintaining stable blood glucose levels.
When Fodmate enzyme activity is insufficient, undigested carbohydrates pass into the large intestine, where gut bacteria ferment them. While some fermentation is normal and even beneficial, excessive fermentation can lead to gas production, bloating, and altered gut motility. Furthermore, the byproducts of this fermentation can influence the gut microbiome composition, potentially affecting overall health in ways researchers are still discovering.
Gut-Brain Axis Interactions
Emerging research suggests that Fodmate enzyme activity may indirectly influence the gut-brain axis, the bidirectional communication system between the central nervous system and the gastrointestinal tract. When carbohydrate digestion is incomplete due to insufficient Fodmate enzyme activity, the resulting bacterial fermentation produces short-chain fatty acids and gases that can trigger intestinal distension and activate visceral sensory pathways.
These signals can influence mood, cognitive function, and even pain perception through neural and hormonal mechanisms. This connection helps explain why digestive discomfort often correlates with psychological symptoms like anxiety or brain fog in conditions like IBS, and why enzyme supplementation sometimes improves both gastrointestinal and neurological symptoms.
Clinical Applications of Fodmate Enzymes
The understanding of Fodmate enzymes has led to numerous clinical applications, from diagnostic tools to therapeutic interventions. These applications continue to expand as research unveils more about these enzymes' functions and potential benefits.
Enzyme Replacement Therapy
Enzyme replacement therapy using Fodmate enzymes has become a standard approach for managing certain digestive disorders. Lactase supplements for lactose intolerance represent the most common example, but other Fodmate enzymes are increasingly available as over-the-counter or prescription supplements. These products provide the missing or insufficient enzymes needed to properly digest specific carbohydrates.
The effectiveness of enzyme replacement therapy varies depending on the specific enzyme, dosage, timing of administration, and individual factors. Generally, these supplements work best when taken just before or with meals containing the problematic carbohydrates. Ongoing research aims to improve enzyme stability, delivery methods, and efficacy to enhance therapeutic outcomes.
Diagnostic Applications
Fodmate enzyme activity levels can serve as valuable diagnostic markers for certain conditions. For instance, measuring lactase activity through breath hydrogen tests helps diagnose lactose intolerance. Similarly, alpha-galactosidase activity tests can identify specific digestive enzyme deficiencies that might contribute to unexplained gastrointestinal symptoms.
Advanced diagnostic techniques now allow for more precise measurement of enzyme activity, helping clinicians differentiate between primary enzyme deficiencies (genetic) and secondary deficiencies (resulting from intestinal damage or disease). This distinction is crucial for determining appropriate treatment approaches and predicting long-term outcomes.
Future Directions in Fodmate Enzyme Research
The field of Fodmate enzyme research is rapidly evolving, with several exciting developments on the horizon that could transform our approach to digestive health and metabolic disorders.
Engineered Enzymes with Enhanced Properties
Protein engineering techniques are being applied to create modified Fodmate enzymes with improved stability, activity, and specificity. These engineered enzymes could overcome limitations of current supplements, such as sensitivity to stomach acid or insufficient activity under physiological conditions. For example, researchers are developing acid-stable lactase variants that remain active throughout the digestive tract, potentially providing more complete lactose digestion.
Another promising direction involves creating broad-spectrum Fodmate enzyme blends that can simultaneously target multiple problematic carbohydrates. Such products could simplify supplementation regimens for individuals with multiple sensitivities and provide more comprehensive digestive support.
Microbiome Modulation
The relationship between Fodmate enzymes and the gut microbiome represents a fascinating area of ongoing research. Rather than simply supplementing with exogenous enzymes, future approaches might focus on modulating the gut microbiome to enhance endogenous enzyme production or compensate for enzyme deficiencies. This could involve targeted probiotics that produce specific Fodmate enzymes or prebiotics that selectively promote beneficial bacterial populations.
Early studies suggest that certain probiotic strains can produce alpha-galactosidase and other Fodmate enzymes, potentially offering a more sustainable approach to addressing enzyme deficiencies. As our understanding of the microbiome-enzyme relationship deepens, more sophisticated interventions will likely emerge.
As research continues to unravel the complexities of Fodmate enzymes and their roles in human metabolism, we can anticipate increasingly personalized approaches to digestive health. From customized enzyme supplements based on individual deficiency profiles to targeted microbiome interventions, the future holds promising possibilities for optimizing metabolic processes and improving quality of life for millions affected by digestive disorders.