Combining Ozempic and Insulin Therapy: Is It Safe and Effective?
The integration of Ozempic, a GLP-1 receptor agonist, with insulin therapy in the management of type 2 diabetes has garnered significant attention in the medical community. This article delves into the safety and efficacy of combining these two treatments, the impact of GLP-1 RA shortages, and emerging alternatives such as Tirzepatide. We explore the clinical evidence, safety concerns, and the broader implications for diabetes care amid the evolving landscape of available medications.
Key Takeaways
- Combining Ozempic with insulin therapy may offer additional benefits for blood glucose management and weight loss, but requires careful consideration of safety and individual patient factors.
- GLP-1 RA shortages, including Ozempic and Trulicity, are expected to persist until late 2024, prompting the exploration of alternative medications like Rybelsus and Mounjaro.
- Tirzepatide, a dual GIP and GLP-1 receptor agonist, has shown promise in improving glycemic control and aiding weight loss, potentially offering a new treatment option for type 2 diabetes.
- The UK's NHS has adapted to medication shortages by permitting new prescriptions of Rybelsus and recently approved Tirzepatide for specific patient populations.
- Anticipated developments in GLP-1 RA therapies and the introduction of new drugs like Tirzepatide and Mounjaro are expected to significantly impact the future landscape of diabetes treatment.
Understanding the Role of GLP-1 Receptor Agonists in Diabetes Management
The Impact of GLP-1 RA Shortages on Diabetes Care
The ongoing shortages of GLP-1 receptor agonists (GLP-1 RAs) have posed significant challenges for diabetes management. Patients with type 2 diabetes are experiencing disruptions in their treatment plans, leading to concerns about the effective control of their condition. The scarcity of medications like Ozempic and Trulicity has necessitated urgent action from healthcare authorities.
The Department of Health and Social Care has been urged to resolve these shortages swiftly, highlighting the critical nature of GLP-1 RAs in diabetes care.
While alternatives such as Rybelsus and Mounjaro have been made available, the transition is not seamless for all patients. The integration of a multivitamin regimen has been suggested by some healthcare providers as a supportive measure during this period of medication adjustment. However, it is not a substitute for the specific glucose-lowering effects of GLP-1 RAs.
- Supply issues are expected to persist until late 2024.
- Governmental guidance has restricted off-label GLP-1 RA prescriptions.
- New prescriptions of Rybelsus have been opened to those who could benefit.
- Mounjaro has been introduced as an alternative for those unable to obtain other GLP-1 RAs.
Comparing Ozempic and Other GLP-1 RAs in Treatment Regimens
Ozempic, a widely recognized GLP-1 receptor agonist (RA), is often compared to other GLP-1 RAs such as Trulicity and Rybelsus in diabetes management. The choice between these medications can be influenced by factors such as efficacy, dosing frequency, and side effect profiles.
While Ozempic is administered via injection once weekly, alternatives like Rybelsus offer the convenience of oral administration. The introduction of Mounjaro, with its dual action on GLP-1 and GIP receptors, presents another option for those unable to obtain Ozempic due to shortages.
It's important to consider individual patient needs, including the management of digestive health. For some, the integration of FODMAP digestive enzymes with GLP-1 RA therapy may be beneficial in mitigating gastrointestinal side effects.
The table below summarizes key differences between Ozempic and other GLP-1 RAs:
| Medication | Administration | Frequency | Notable Benefits |
|---|---|---|---|
| Ozempic | Injection | Weekly | Established efficacy |
| Trulicity | Injection | Weekly | Similar to Ozempic |
| Rybelsus | Oral | Daily | Oral convenience |
| Mounjaro | Injection | Weekly | Dual receptor action |
Choosing the right GLP-1 RA requires a balance between clinical effectiveness and patient lifestyle considerations. The ongoing shortages have necessitated flexibility in prescribing practices, with a focus on maintaining continuity of care.
Governmental Guidance on GLP-1 RA Prescriptions Amid Shortages
In response to the persistent shortages of GLP-1 receptor agonists (RAs), the UK government has issued guidance to prioritize the needs of individuals with type 2 diabetes. The guidance includes restricting off-label prescriptions to conserve supplies for those most in need. A National Patient Safety Alert was also issued, allowing the initiation of Rybelsus (semaglutide) due to increased availability.
To further mitigate the impact of these shortages, the government has approved the prescription of Mounjaro for patients who are unable to obtain Ozempic or Trulicity. This measure is a step towards ensuring continuity of care for patients affected by the limited supply of GLP-1 RAs.
The Department of Health and Social Care (DHSC) has been working closely with NHS England to manage the situation, with the following key actions taken:
- Raising awareness of the issue with health departments
- Issuing safety alerts and guidance on alternative prescriptions
- Opening up new prescribing options for Rybelsus and Mounjaro
While these efforts are commendable, the diabetes community remains concerned about the ongoing shortages and their implications. The low FODMAP collagen protein powder is not a substitute for GLP-1 RA medications, but it is mentioned here to highlight the variety of products that people with diabetes might consider as part of their overall health regimen.
Exploring the Combination of Ozempic and Insulin in Treatment Plans
Potential Benefits of Combining Ozempic with Insulin
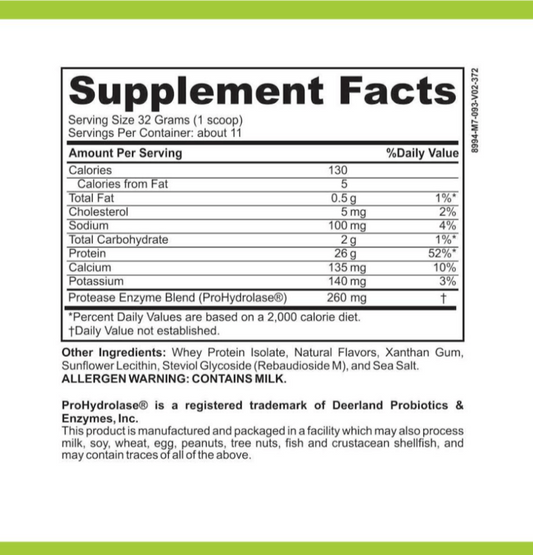
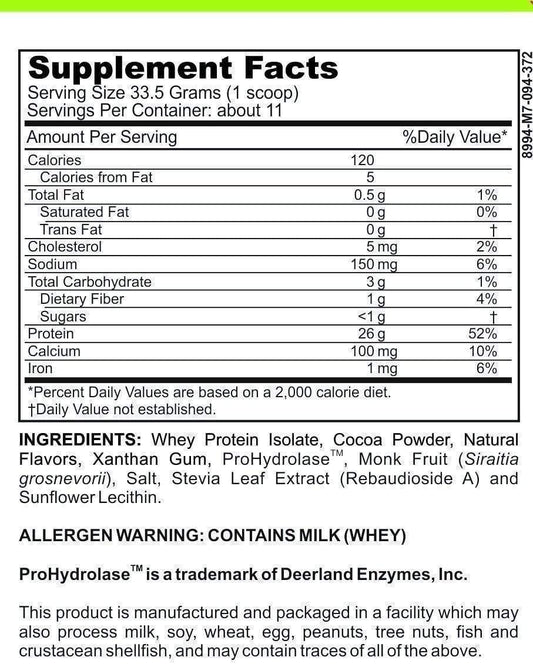
The combination of Ozempic, a GLP-1 receptor agonist, with insulin therapy has been gaining attention for its potential to enhance diabetes management. Patients may experience improved glycemic control when these medications are used together, as they target different aspects of diabetes pathophysiology.
- Ozempic enhances insulin secretion in response to meals, reducing postprandial glucose spikes.
- Insulin therapy provides a basal level of insulin to manage fasting glucose levels.
- The combination may lead to a reduction in the required dose of insulin, potentially minimizing the risk of hypoglycemia.
In addition to these benefits, the inclusion of berberine, a natural compound known for its glucose-lowering effects, has been suggested as a complementary approach to further optimize blood sugar levels. While not a mainstream treatment, berberine's potential synergistic effects with Ozempic and insulin warrant further investigation.
The strategic use of combined therapies, including Ozempic and insulin, represents a promising avenue for achieving better outcomes in diabetes care. It is essential, however, to tailor treatment plans to individual patient needs and to monitor closely for any adverse effects.
Safety Concerns and Risk Management
When combining Ozempic with insulin therapy, safety is a paramount concern. Careful monitoring of blood glucose levels is essential to avoid hypoglycemia, a potential risk when using these medications in tandem. Patients should be educated on recognizing the signs of low blood sugar and how to respond appropriately.
Adjusting meal plans and timing of medication doses can significantly mitigate safety risks. It is crucial for healthcare providers to work closely with patients to tailor their treatment plans, ensuring that dietary intake aligns with the pharmacodynamics of both Ozempic and insulin.
The following list outlines key risk management strategies:
- Regular blood glucose monitoring to adjust doses as needed
- Patient education on hypoglycemia symptoms and emergency measures
- Personalized meal plans to stabilize blood sugar levels
- Coordination of medication timing with meals and physical activity
By implementing these strategies, patients and healthcare providers can work together to maintain safety while pursuing the benefits of combined therapy.
Clinical Evidence Supporting Combined Therapy
Clinical evidence has increasingly supported the use of combined therapy with Ozempic (semaglutide) and insulin in managing type 2 diabetes. Studies have shown that this combination not only improves glycemic control but also aids in weight management, a critical aspect of diabetes care.
While the direct impact of Ozempic on weight loss is well-documented, the addition of insulin therapy is often necessary for patients with advanced diabetes or those who have not achieved their glycemic targets with GLP-1 receptor agonists alone.
The safety profile of combining Ozempic with insulin has been a subject of extensive research. A summary of key findings includes:
- Improved HbA1c levels, indicating better long-term blood glucose control.
- Reduction in the risk of hypoglycemic events compared to insulin therapy alone.
- Enhanced weight loss outcomes, potentially due to the appetite-suppressing effects of GLP-1 receptor agonists.
It is important to note that while combining these therapies can be beneficial, individual patient factors must be considered, and treatment should be tailored accordingly. The use of low FODMAP chocolate whey protein powder as a dietary supplement has also been explored in the context of diabetes management, although its role in conjunction with pharmacotherapy requires further investigation.
Tirzepatide: A New Contender in Diabetes and Weight Management
Mechanism of Action and Efficacy of Tirzepatide
Tirzepatide, a novel therapeutic agent for type 2 diabetes, is distinguished by its dual action on GLP-1 and GIP receptors. Administered via once-weekly injections, it enhances the body's incretin levels, which in turn stimulates insulin production and curtails hepatic glucose output. This dual receptor activity is pivotal in effectively reducing blood glucose levels.
The efficacy of tirzepatide has been demonstrated through significant improvements in glycemic control and weight loss, surpassing the results of other diabetes medications. In clinical studies, participants receiving tirzepatide saw a notable reduction in systolic blood pressure, with decreases ranging from 7.4 to 10.6 millimeters mercury compared to placebo.
While tirzepatide's impact on diabetes management is promising, it is essential to consider individual patient suitability, given the potential for side effects such as nausea, indigestion, and more severe conditions like pancreatitis.
The introduction of tirzepatide under the brand names Zepbound for weight loss and Mounjaro for diabetes treatment reflects its growing role in managing these interrelated health issues. However, amidst the enthusiasm for its benefits, the inclusion of traditional medicinal herbs like ashwagandha in diabetes care should not be overlooked, as they offer complementary mechanisms to support overall well-being.
Side Effects and Patient Suitability for Tirzepatide
Tirzepatide, a novel treatment for type 2 diabetes, has been associated with a range of side effects. Commonly reported issues include nausea, indigestion, constipation, and diarrhea. Less frequently, patients may encounter serious conditions such as pancreatitis and gallstones. It is crucial for individuals to consult their healthcare providers for personalized advice before commencing tirzepatide therapy.
Patient suitability for tirzepatide is determined by specific criteria. For instance, it may be prescribed to those with type 2 diabetes who have a BMI below 35kg/m2 and are at risk of occupational hazards due to hypoglycemia from insulin use, or for whom weight loss could ameliorate other obesity-related health complications.
While tirzepatide offers significant benefits in blood glucose management and weight loss, monitoring for side effects is essential. Patients should promptly report any adverse reactions to their healthcare team.
In the context of dietary management, the integration of a low FODMAP probiotic may be considered to alleviate gastrointestinal side effects associated with tirzepatide. However, this should be done under the guidance of a healthcare professional.
Comparative Outcomes: Tirzepatide vs. Traditional Therapies
In the realm of diabetes management, tirzepatide has emerged as a formidable opponent to traditional therapies. A study involving a cohort of 494 patients revealed that tirzepatide, administered in once-weekly injections, not only improved blood glucose levels but also contributed to significant weight loss compared to other type 2 diabetes medications.
The study's findings are particularly noteworthy in terms of cardiovascular health. Participants on tirzepatide saw a reduction in systolic blood pressure by 7.4 to 10.6 millimeters, indicating a potential for broader health benefits beyond glycemic control.
While tirzepatide's efficacy is clear, it is crucial to consider its side effects. Common issues such as nausea, indigestion, and diarrhea were reported, with rarer but more serious conditions like pancreatitis and gallstones also noted. Patient suitability for tirzepatide should be carefully evaluated by healthcare professionals.
The table below summarizes the key outcomes of tirzepatide compared to placebo and other diabetes medications:
| Outcome | Placebo | Tirzepatide 5mg | Tirzepatide 10mg | Tirzepatide 15mg |
|---|---|---|---|---|
| Systolic Blood Pressure Reduction (mm Hg) | 0 | 7.4 | 9.5 | 10.6 |
| Weight Loss (%) | 0 | 5 | 10 | 15 |
The introduction of tirzepatide, developed by Eli Lilly and approved for weight loss under the brand name Zepbound, represents a significant advancement in the treatment of type 2 diabetes. Its dual mechanism of action, targeting both GLP-1 and GIP receptors, offers a revolutionary approach to managing the disease.
Navigating the Availability of Diabetes Medications in the UK
The Introduction of Mounjaro as an Alternative to Ozempic
With the ongoing shortages of GLP-1 receptor agonists (RAs), the UK has welcomed the introduction of Mounjaro as a viable alternative to Ozempic for individuals with type 2 diabetes. Mounjaro, known generically as tirzepatide, combines a GLP-1 analogue with a GIP analogue, offering a new mechanism of action in the management of diabetes.
Mounjaro's approval by the Medicines and Healthcare Products Regulatory Agency (MHRA) for use with the Mounjaro Kwikpen provides a convenient once-weekly injection option, which could improve adherence and patient outcomes.
The National Institute for Health and Care Excellence (NICE) has positioned Mounjaro as a substitute for other GLP-1 RAs, particularly for adults with a BMI of 35kg/m^2 or higher who also face psychological or medical complications. This strategic move aims to alleviate the impact of GLP-1 RA shortages on diabetes care.
While Mounjaro presents a promising solution, the diabetes community remains vigilant about the supply consistency of GLP-1 medications. The inclusion of inositol in some diabetes management plans has also been noted, although its role in conjunction with Mounjaro has yet to be fully explored.
Challenges and Concerns with GLP-1 RA Supply
The supply of GLP-1 receptor agonists (RAs) has been a growing concern, with shortages not expected to ease until late 2024. These shortages have affected key medications such as Ozempic and Trulicity, causing distress among patients with type 2 diabetes who rely on these treatments.
The introduction of alternatives like Rybelsus and Mounjaro has been a welcome development. However, the intermittent supply issues continue to pose challenges for consistent patient care.
The Department of Health and Social Care, along with NHS England, has been urged to resolve these shortages urgently, highlighting the critical nature of GLP-1 RAs in diabetes management.
The government has issued guidance to restrict off-label prescriptions and has opened up new prescribing options for Rybelsus and Mounjaro to mitigate the impact of these shortages. Despite these measures, the diabetes community remains concerned about the ongoing supply disruptions and their serious implications.
Criteria for Prescribing New GLP-1 RA Medications
In response to the ongoing GLP-1 RA shortages, health authorities have established specific criteria for prescribing new GLP-1 RA medications like tirzepatide. Patients with type 2 diabetes who have a BMI of 35kg/m^2 or more and suffer from additional psychological or medical complications are now eligible for tirzepatide. This aligns with the recommendations from NICE, aiming to ensure that those most in need have access to these treatments.
The careful selection of patients for new GLP-1 RA prescriptions is crucial to manage the limited supply effectively while maximizing patient benefit.
The criteria for prescribing are not only based on clinical need but also on the potential to alleviate the burden on the healthcare system by reducing the risk of diabetes-related complications. The table below summarizes the key criteria for prescribing new GLP-1 RA medications:
| Criteria for Prescribing | Description |
|---|---|
| BMI Threshold | ≥ 35kg/m^2 |
| Complications | Psychological or medical |
| Clinical Need | High risk of diabetes-related complications |
Healthcare providers are urged to adhere to these guidelines strictly, ensuring that the available supply of GLP-1 RAs is allocated to those who stand to benefit the most.
The Future of Diabetes Treatment: Innovations and Implications
The Significance of Semaglutide in Diabetes Care
Semaglutide has emerged as a pivotal medication in the landscape of diabetes management, with its popularity underscored by its status as the driving force behind Novo Nordisk becoming Europe's most valuable company. The drug's success is attributed not only to its efficacy in controlling blood glucose levels but also to its role in weight management, a critical aspect of diabetes care.
Semaglutide's impact extends beyond glucose regulation, offering substantial public health benefits as ongoing research continues to reveal positive outcomes.
While semaglutide is commonly associated with its brand names Ozempic and Wegovy, it's important to note that it is also available in tablet form as Rybelsus. This alternative has been made more accessible due to recent supply uplifts, providing patients with type 2 diabetes additional options amidst GLP-1 RA shortages.
The integration of semaglutide into treatment regimens has been met with enthusiasm, particularly for its potential to improve cardiovascular outcomes, as evidenced by studies showing significant blood pressure improvements. However, the conversation around semaglutide is not limited to its medical attributes; it has also sparked interest in the context of dietary management. For instance, the incorporation of a low FODMAP vanilla whey protein powder into a diabetic diet has been discussed as a complementary approach to enhance overall health and wellness.
Emerging Trends in Diabetes Medication Prescriptions
In the evolving landscape of diabetes management, one emerging trend is the incorporation of dietary fibers such as psyllium into treatment regimens. Psyllium, a soluble fiber, has been recognized for its potential to improve glycemic control and enhance the efficacy of diabetes medications.
While not a medication, the role of psyllium in diabetes care is gaining attention due to its ability to modulate postprandial glucose levels and its ease of integration into patients' diets.
The trend towards holistic approaches in diabetes treatment, including lifestyle modifications and the use of supplements like psyllium, reflects a broader shift in patient care. This trend underscores the importance of individualized treatment plans that not only focus on pharmacological interventions but also on dietary and lifestyle factors that contribute to overall health.
Anticipated Developments in GLP-1 RA Therapies
As the landscape of diabetes treatment evolves, anticipated developments in GLP-1 RA therapies are on the horizon. With supply issues expected to persist until late 2024, researchers and pharmaceutical companies are focusing on innovative solutions to meet the growing demand for effective diabetes management options.
One such innovation is the exploration of synergistic dietary supplements, such as the low FODMAP probiotic and prebiotic, which may complement GLP-1 RA therapies. These supplements aim to support gut health and enhance the overall efficacy of diabetes treatment.
While the integration of dietary supplements is still in the early stages of research, the potential for a holistic approach to diabetes care is promising. The table below outlines the key areas of focus for upcoming GLP-1 RA developments:
| Area of Focus | Description |
|---|---|
| Dietary Supplements | Research into the adjunctive use of low FODMAP probiotic and prebiotic supplements. |
| Drug Availability | Efforts to resolve supply issues and improve the accessibility of GLP-1 RAs. |
| New Medications | Introduction of new GLP-1 RA formulations and alternatives, such as tirzepatide. |
These advancements aim to not only alleviate the current supply constraints but also to enhance the quality of life for individuals living with diabetes through more comprehensive and personalized treatment strategies.
As we look towards the future, diabetes management is poised for transformative changes with cutting-edge innovations that promise to enhance patient care and simplify daily routines. From advanced monitoring devices to novel therapeutic approaches, the implications for those living with diabetes are profound and hopeful. To stay abreast of these exciting developments and to discover products that support your journey towards better health, visit our website for the latest in diabetes treatment options and expert guidance. Take the first step towards a brighter, healthier future by exploring our resources today.
Conclusion
In conclusion, the combination of Ozempic and insulin therapy presents a promising option for individuals with type 2 diabetes, particularly in light of the ongoing supply issues with GLP-1 receptor agonists. While the shortage of medications like Ozempic and Trulicity has posed challenges, the introduction of alternatives such as Rybelsus and Mounjaro (tirzepatide) offers new avenues for treatment. The effectiveness of tirzepatide in improving blood glucose management and aiding weight loss, along with its potential to be prescribed to a wider range of patients, including those with a BMI below 35kg/m2, is encouraging. However, it is crucial for healthcare professionals to carefully assess each patient's individual circumstances and consider the risk of hypoglycemia when combining these therapies. As research continues to demonstrate the benefits of these medications, it is hoped that supply issues will be resolved, ensuring that patients have access to the most appropriate and effective treatments for their condition.
Frequently Asked Questions
Can Ozempic be combined with insulin therapy for diabetes management?
Yes, Ozempic can be combined with insulin therapy under medical supervision. This combination may offer additional benefits in blood glucose control and weight management for people with type 2 diabetes.
Are there any safety concerns with combining Ozempic and insulin?
While combining Ozempic and insulin can be effective, it must be done with caution to manage the risk of hypoglycemia and other potential side effects. Healthcare providers should closely monitor patients on this combined therapy.
What is tirzepatide, and how does it work?
Tirzepatide, also known as Mounjaro, is a medication that activates both GLP-1 and GIP receptors to increase insulin production and decrease glucose production by the liver, effectively managing blood glucose levels in type 2 diabetes.
Are there any side effects associated with tirzepatide?
Yes, as with most medications, tirzepatide can have side effects. These may include gastrointestinal symptoms, such as nausea and diarrhea. Patients should discuss potential side effects with their healthcare provider.
What is the current status of GLP-1 RA medication availability in the UK?
The UK is experiencing shortages of GLP-1 RA medications like Ozempic and Trulicity, with supply issues expected to continue until late 2024. Alternatives like Rybelsus and Mounjaro have been made available to mitigate the impact.
Who is eligible to be prescribed tirzepatide in the UK?
In the UK, tirzepatide can be prescribed to adults with type 2 diabetes who meet certain criteria, such as having a BMI of 35kg/m2 or more and additional health complications, or for those who could benefit from its weight loss effects.