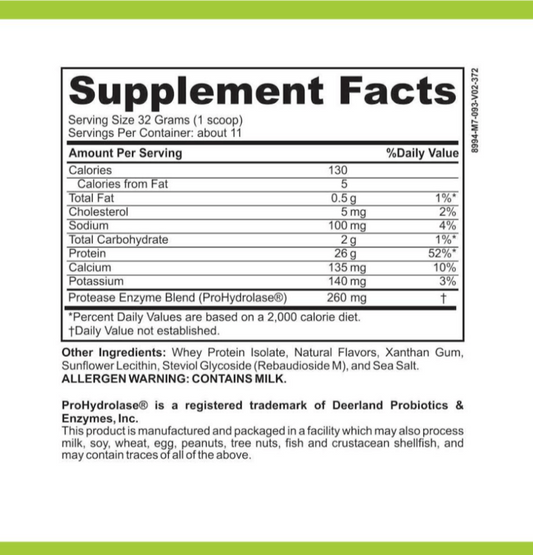
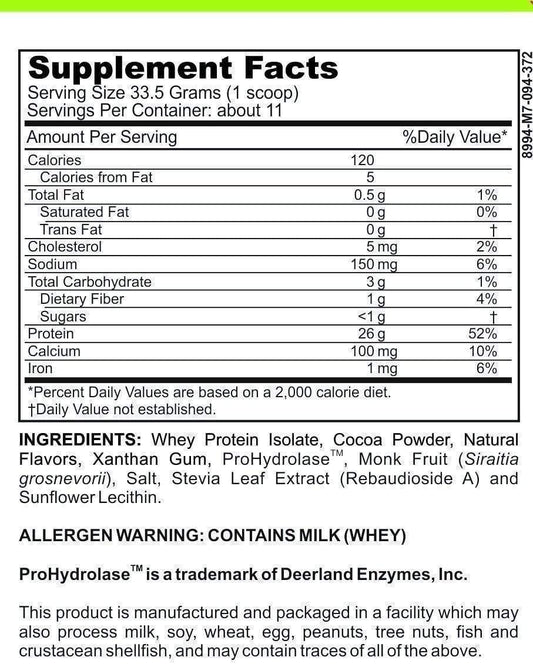
LRA Professional's Choice 389 Panel by ELISA / ACT Biotechnologies Vs Cytometric Assay
LRA Professional's Choice 389 Panel by ELISA / ACT Biotechnologies Vs Cytometric Assay
In the field of medical research and diagnosis, there are various methods and technologies that assist in understanding and unraveling complex diseases. Two such methods are the LRA Professional's Choice 389 Panel by ELISA / ACT Biotechnologies and Cytometric Assay. This article aims to provide an in-depth understanding of both these methods, compare their features, methodologies, and discuss their practical applications.
Understanding the LRA Professional's Choice 389 Panel
The LRA Professional's Choice 389 Panel is an innovative diagnostic tool designed to identify delayed hypersensitivity reactions in patients. Developed by ELISA / ACT Biotechnologies, this panel revolutionizes the way we approach medical diagnoses. By systematically testing the patient's immune response to a wide range of substances, it provides valuable insights into factors affecting their overall health.
Delayed hypersensitivity reactions can often go unnoticed or misdiagnosed, leading to prolonged suffering for patients. The LRA Professional's Choice 389 Panel aims to address this issue by offering a comprehensive evaluation of the patient's immune system. It goes beyond traditional diagnostic methods by focusing on delayed immune reactions, which play a crucial role in chronic illnesses.
The Role of ELISA / ACT Biotechnologies in LRA Panel
ELISA / ACT Biotechnologies is a leader in advanced diagnostic technologies, and their contribution to the LRA Professional's Choice 389 Panel is groundbreaking. With years of research and expertise in the field, they have developed a comprehensive method to analyze delayed immune reactions and provide accurate results.
By harnessing the power of ELISA (enzyme-linked immunosorbent assay) and ACT (antigen-specific lymphocyte stimulation test) technologies, ELISA / ACT Biotechnologies has paved the way for a deeper understanding of immune system dysfunctions. Their cutting-edge approach allows healthcare professionals to identify and address the root causes of various health issues.
Key Features of the LRA Professional's Choice 389 Panel
The LRA Professional's Choice 389 Panel offers several key features that set it apart from traditional diagnostic methods. Firstly, it tests for delayed hypersensitivity, which plays a crucial role in chronic illnesses. This focus on delayed immune reactions allows for a comprehensive evaluation of the patient's immune system.
With the ability to identify reactions to a wide range of substances, including foods, environmental factors, and chemicals, the LRA Professional's Choice 389 Panel ensures that potential triggers are not overlooked during the diagnostic process. This broad coverage is essential in providing a holistic understanding of the patient's immune response and potential allergens.
Furthermore, the LRA Professional's Choice 389 Panel offers a detailed analysis of the patient's immune system by measuring both IgG and IgE antibodies. This dual approach provides a more comprehensive picture of the immune response, allowing healthcare professionals to tailor treatment plans accordingly.
Another notable feature of the LRA Professional's Choice 389 Panel is its ability to detect delayed hypersensitivity reactions to environmental factors. This includes pollutants, molds, and other common allergens that can have a significant impact on an individual's health. By identifying these triggers, healthcare professionals can help patients make informed lifestyle changes and create a healthier living environment.
In conclusion, the LRA Professional's Choice 389 Panel, developed by ELISA / ACT Biotechnologies, is a groundbreaking diagnostic tool that offers a comprehensive evaluation of delayed hypersensitivity reactions. With its advanced technologies and broad coverage, it provides healthcare professionals with valuable insights into the patient's immune system and potential allergens. By understanding the intricacies of immune system dysfunctions, healthcare professionals can develop personalized treatment plans to improve patient outcomes.
Introduction to Cytometric Assay
Cytometric Assay, on the other hand, is a powerful method used to analyze cellular characteristics and functions. It involves the use of flow cytometry, a technique that allows for the measurement and sorting of cells based on their unique properties. This method has been instrumental in various areas of medical research.
Flow cytometry, the cornerstone of cytometric assay, is a sophisticated technology that has revolutionized the field of cell analysis. It combines principles of fluid dynamics, optics, and immunology to provide researchers with a wealth of information about cellular populations. By harnessing the power of lasers and fluorescent labels, flow cytometry enables scientists to delve deep into the intricacies of cells and unravel their mysteries.
The Principle of Cytometric Assay
At its core, cytometric assay relies on the detection and measurement of cellular markers using fluorescent labels. By targeting specific molecules on the surface or within the cells, researchers can gain insights into the cells' behavior and functions. This technology enables the analysis of thousands of cells rapidly, providing a holistic view of cellular populations.
Fluorescent labels, also known as fluorochromes, are compounds that emit light of a specific wavelength when exposed to a particular excitation source. These labels can be attached to antibodies, DNA probes, or other molecules that specifically bind to cellular targets of interest. When a cell passes through the flow cytometer, it is illuminated by laser beams, causing the fluorochromes to emit fluorescence. The emitted light is then collected and analyzed, allowing researchers to determine the presence and quantity of the target molecules.
Applications of Cytometric Assay in Medical Research
Cytometric assay has a wide range of applications in medical research. It has been pivotal in understanding the immune system's response to infections, analyzing cancerous cells, and studying cellular changes in various diseases. Additionally, it allows researchers to assess drug efficacy, evaluate stem cell populations, and even monitor the progression of AIDS. The versatility and accuracy of cytometric assay make it an indispensable tool in the medical research community.
One of the key applications of cytometric assay is in immunology research. By using specific fluorescently labeled antibodies, researchers can identify and quantify different immune cell populations. This information helps in understanding the dynamics of immune responses, such as the activation and proliferation of T cells, the production of cytokines, and the interaction between immune cells and pathogens.
In cancer research, cytometric assay plays a crucial role in characterizing tumor cells and monitoring disease progression. By analyzing the expression of specific cell surface markers, researchers can classify cancer cells into different subtypes and determine their aggressiveness. This information aids in the development of targeted therapies and personalized treatment strategies.
Furthermore, cytometric assay has proven invaluable in the study of various diseases, such as autoimmune disorders, cardiovascular diseases, and neurological conditions. By examining cellular changes and aberrant protein expression, researchers can gain insights into the underlying mechanisms of these diseases and identify potential therapeutic targets.
Not limited to disease research, cytometric assay also finds applications in drug development and clinical diagnostics. It allows researchers to evaluate the efficacy and toxicity of potential drugs on cellular populations, enabling the identification of promising candidates for further development. In clinical diagnostics, cytometric assay can be used to detect and monitor specific disease markers, aiding in early diagnosis and disease management.
In conclusion, cytometric assay, powered by flow cytometry, has revolutionized the field of cell analysis and has become an indispensable tool in medical research. Its ability to rapidly analyze thousands of cells and provide detailed information about cellular populations has paved the way for groundbreaking discoveries and advancements in various areas of medicine. As technology continues to evolve, cytometric assay will undoubtedly play an even more significant role in unraveling the complexities of the cellular world.
Comparing ELISA / ACT Biotechnologies and Cytometric Assay
While both the LRA Professional's Choice 389 Panel and cytometric assay provide valuable insights into the human immune system and cellular behavior, there are notable differences between the two methods. Understanding these distinctions can help researchers and medical professionals choose the most appropriate approach for diverse scenarios.
Methodology Comparison: ELISA / ACT Biotechnologies Vs Cytometric Assay
The LRA Professional's Choice 389 Panel utilizes ELISA-based techniques to detect delayed immune reactions. This enzyme-linked immunosorbent assay enables highly specific and sensitive measurements, providing reliable results. On the other hand, cytometric assay employs flow cytometry, allowing for the simultaneous analysis of multiple cell parameters. Both methods have their strengths, and the choice depends on the specific objectives of the research or diagnosis.
Accuracy and Precision: A Comparative Analysis
When it comes to accuracy and precision, both the LRA Professional's Choice 389 Panel and cytometric assay excel in their respective domains. The ELISA-based approach of the LRA panel ensures accurate identification of delayed immune reactions. On the other hand, cytometric assay provides precise measurements of cellular markers, offering valuable quantitative data. It is essential to consider the specific requirements of the study or diagnosis to determine which method is best suited for the given scenario.
Case Studies and Practical Applications
Real-life case studies highlight the effectiveness and practical applications of both the LRA Professional's Choice 389 Panel and cytometric assay. These studies serve as examples of how these methods have been utilized in different medical scenarios, further reinforcing their value in the field.
Case Study: Using the LRA Professional's Choice 389 Panel in Clinical Diagnosis
In a clinical setting, the LRA Professional's Choice 389 Panel has proven to be an invaluable tool in identifying underlying causes of chronic conditions. For instance, a patient presenting with persistent gastrointestinal issues underwent comprehensive testing with the LRA panel. The results revealed delayed immune reactions to certain food items, leading to a tailored dietary intervention. This case study demonstrates the potential of the LRA panel in personalized medicine and improving patient outcomes.
Case Study: Cytometric Assay in Disease Research
In disease research, cytometric assay has played a pivotal role. For example, researchers studying the immune response to a specific pathogen used cytometric assay to identify and analyze different immune cell subsets. The data obtained helped elucidate the intricate mechanisms underlying the body's defense against the pathogen. This case study showcases the power of cytometric assay in advancing our understanding of disease processes.
Future Perspectives and Conclusions
As medical research continues to progress, it is crucial to consider the future perspectives and potential advancements in both the LRA Professional's Choice 389 Panel and cytometric assay.
Advancements and Innovations in ELISA / ACT Biotechnologies
ELISA / ACT Biotechnologies is at the forefront of diagnostic advancements. They continuously strive to improve the accuracy and efficiency of their LRA panel, incorporating feedback from researchers and medical professionals. Ongoing research may lead to expanded panel options and increased diagnostic capabilities, further enhancing patient care.
The Future of Cytometric Assay in Biomedical Research
Cytometric assay holds tremendous promise for future biomedical research. The continued development of novel fluorescent labels, improved instrumentation, and data analysis techniques will contribute to even more precise and comprehensive analyses. These advancements will propel our understanding of cellular behavior and aid in the development of targeted therapies.
Final Thoughts on the Comparison and Use of Both Methods
In conclusion, the LRA Professional's Choice 389 Panel by ELISA / ACT Biotechnologies and cytometric assay offer unique capabilities and numerous applications in the field of medical research and diagnosis. The LRA panel excels in identifying delayed immune reactions, providing valuable insights into patients' overall health status. On the other hand, cytometric assay enables researchers to study cellular properties with remarkable precision. The choice between these methods depends on specific research objectives and diagnostic requirements. Embracing the strengths of both these approaches can unlock a wealth of information, leading to improved patient care and advancements in medical science.