LRA Comprehensive with Medications 349 Panel by ELISA / ACT Biotechnologies Vs Cytometric Assay
LRA Comprehensive with Medications 349 Panel by ELISA / ACT Biotechnologies Vs Cytometric Assay
The field of medical testing has seen significant advancements in recent years, offering improved diagnostic tools and techniques. Among these advancements is the LRA Comprehensive with Medications 349 Panel, which utilizes two different methods for analysis: ELISA / ACT Biotechnologies and Cytometric Assay. In this article, we will delve into the basics of this panel and explore the differences and similarities between these two testing methods.
Understanding the Basics of LRA Comprehensive with Medications 349 Panel
What is LRA Comprehensive with Medications 349 Panel?
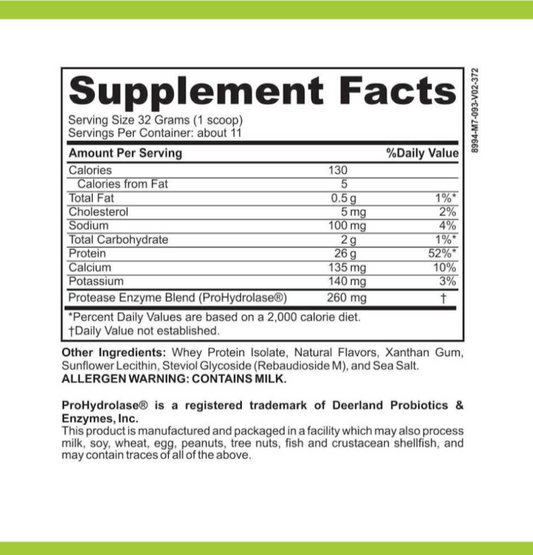
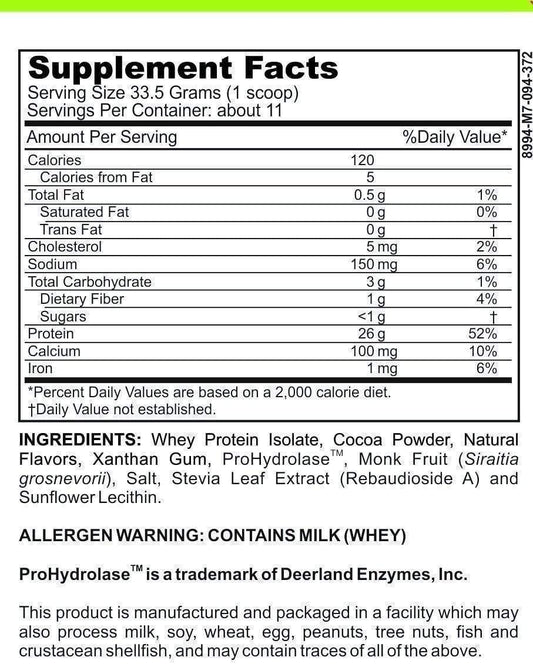
LRA Comprehensive with Medications 349 Panel is a comprehensive diagnostic panel that aims to identify delayed hypersensitivity reactions to various substances, including foods, environmental factors, and medications. This panel goes beyond traditional allergy testing by assessing a broad range of immune responses, providing valuable insights into the body's overall reactivity.
Delayed hypersensitivity reactions are immune responses that occur several hours or even days after exposure to an antigen. These reactions can manifest in various ways, such as skin rashes, digestive issues, joint pain, and fatigue. Identifying the specific triggers behind these reactions is crucial for developing an effective treatment plan.
The LRA Comprehensive with Medications 349 Panel is designed to provide a comprehensive analysis of the body's immune response to a wide range of substances. It includes testing for over 300 food antigens, environmental factors such as pollen and mold, and a comprehensive medication panel consisting of 349 different medications.
By testing for such a wide array of substances, the LRA Comprehensive with Medications 349 Panel offers a more complete picture of an individual's immune reactivity. This can help healthcare professionals identify potential triggers for a patient's symptoms and develop personalized treatment strategies.
How Does LRA Comprehensive with Medications 349 Panel Work?
The LRA Comprehensive with Medications 349 Panel works by measuring the body's immune response to specific antigens. It is a form of lymphocyte response assay (LRA), which involves the stimulation of peripheral blood mononuclear cells (PBMCs) with antigens in vitro. The immune reaction is then assessed by various methods, such as ELISA / ACT Biotechnologies and Cytometric Assay.
During the LRA process, a blood sample is collected from the patient and sent to a specialized laboratory for analysis. The laboratory technicians isolate the peripheral blood mononuclear cells, which include lymphocytes and monocytes, from the sample. These cells play a crucial role in the body's immune response.
The isolated cells are then exposed to a panel of antigens, including the 349 medications included in the LRA Comprehensive with Medications Panel. The antigens stimulate the immune cells, triggering a cascade of immune responses. The laboratory technicians carefully monitor and measure these responses to determine the level of reactivity to each antigen.
ELISA (enzyme-linked immunosorbent assay) and Cytometric Assay are two common methods used to assess the immune response in the LRA Comprehensive with Medications 349 Panel. These techniques involve the use of specific antibodies that can detect and quantify the presence of immune markers, such as cytokines and immunoglobulins, which are produced during an immune response.
By analyzing the immune markers produced by the stimulated cells, the laboratory can determine the level of reactivity to each antigen. This information is then compiled into a comprehensive report, which provides healthcare professionals with valuable insights into the patient's immune reactivity to various substances.
It is important to note that the LRA Comprehensive with Medications 349 Panel is a specialized test that requires the expertise of trained healthcare professionals and specialized laboratories. The results of this panel should be interpreted by qualified professionals who can integrate the findings into a comprehensive treatment plan.
Delving into ELISA / ACT Biotechnologies
The Role of ELISA in LRA Comprehensive with Medications 349 Panel
ELISA, or enzyme-linked immunosorbent assay, is a widely used technique in medical testing. In the context of LRA Comprehensive with Medications 349 Panel, ELISA is employed to detect and quantify specific antibodies produced in response to antigens. This method utilizes the principle of antigen-antibody interaction, allowing for accurate measurement of immune response levels.
When a patient undergoes the LRA Comprehensive with Medications 349 Panel, their blood sample is collected and processed in a laboratory. The ELISA test begins by coating a microplate with the specific antigens of interest. These antigens can be derived from various sources, such as food, environmental allergens, or medications. Once the antigens are immobilized on the microplate, the patient's serum is added.
The patient's serum contains antibodies that may have been produced as a result of exposure to certain antigens. If these antibodies are present in the serum, they will bind to the corresponding antigens on the microplate. This binding is the crucial step in ELISA, as it forms the basis for the subsequent detection and quantification of the antibodies.
After allowing sufficient time for the antibodies to bind, the microplate is washed to remove any unbound substances. Then, an enzyme-linked secondary antibody is added. This secondary antibody recognizes and binds to the patient's antibodies that are already bound to the antigens on the microplate.
The secondary antibody is conjugated to an enzyme, such as horseradish peroxidase or alkaline phosphatase. This enzyme acts as a catalyst for a color-producing reaction. A substrate specific to the enzyme is added, and if the enzyme is present, it will convert the substrate into a colored product. The intensity of the color is directly proportional to the amount of antibodies present in the patient's serum.
The color development is measured using a spectrophotometer, which quantifies the absorbance of light at a specific wavelength. By comparing the absorbance of the patient's sample to a standard curve generated using known concentrations of antibodies, the concentration of antibodies in the patient's serum can be determined.
Exploring the Functionality of ACT Biotechnologies
ACT Biotechnologies, on the other hand, is a leading provider of innovative diagnostic solutions. Their approach within the LRA Comprehensive with Medications 349 Panel utilizes cutting-edge technologies to analyze immune reactions at a cellular level. By measuring the activation of specific cells, ACT Biotechnologies provides valuable insights into the patient's immune system reactivity.
ACT Biotechnologies employs a technique called lymphocyte activation testing (LAT) to assess the immune response of a patient. This test evaluates the activation of lymphocytes, a type of white blood cell that plays a crucial role in the immune system's response to antigens.
When a patient's blood sample is processed for LAT, the lymphocytes are isolated and exposed to a panel of antigens. These antigens can be derived from a wide range of sources, including foods, environmental factors, and medications. The lymphocytes are then monitored for signs of activation, such as changes in size, shape, or the release of specific molecules.
ACT Biotechnologies utilizes advanced flow cytometry techniques to analyze the activation of lymphocytes. Flow cytometry allows for the simultaneous measurement of multiple parameters, such as cell size, granularity, and the expression of specific molecules on the cell surface. By analyzing these parameters, ACT Biotechnologies can identify which antigens trigger an immune response in the patient.
Furthermore, ACT Biotechnologies goes beyond traditional LAT by incorporating additional markers to assess the functional capacity of lymphocytes. These markers provide insights into the specific immune pathways that are activated upon exposure to antigens. By understanding the functional capacity of the immune system, healthcare professionals can tailor treatment plans to address any deficiencies or overreactions.
The data generated by ACT Biotechnologies' analysis is compiled into comprehensive reports that provide detailed information about the patient's immune system reactivity. These reports assist healthcare professionals in making informed decisions regarding treatment options, dietary modifications, and environmental changes that may benefit the patient's overall health and well-being.
Cytometric Assay: An Overview
The Principle of Cytometric Assay
Cytometric Assay, also known as flow cytometry, is another method employed within the LRA Comprehensive with Medications 349 Panel. This technique involves the analysis of cells in a fluid sample, allowing for the identification and quantification of specific cellular markers or proteins. It offers a robust approach to assess immune responses in a comprehensive and efficient manner.
The Application of Cytometric Assay in Medical Testing
In the context of the LRA Comprehensive with Medications 349 Panel, cytometric assay enables the identification and characterization of specific cell populations involved in immune responses. By analyzing these cells, healthcare professionals can gain valuable insights into the patient's immune system and identify potential triggers for allergic or hypersensitivity reactions.
ELISA / ACT Biotechnologies Vs Cytometric Assay: A Comparative Analysis
Strengths and Limitations of ELISA / ACT Biotechnologies
ELISA and ACT Biotechnologies offer several advantages in the LRA Comprehensive with Medications 349 Panel. These methods provide accurate measurements of immune responses, allowing for precise diagnostic information. However, they may not capture all types of immune reactions and may have limitations in assessing complex hypersensitivity responses.
Advantages and Disadvantages of Cytometric Assay
Cytometric Assay, on the other hand, offers a comprehensive analysis of cellular-level immune responses. It can identify and characterize specific immune cell populations, providing a more detailed understanding of the immune system. However, this method may require more specialized equipment and expertise for analysis.
Key Differences and Similarities Between ELISA / ACT Biotechnologies and Cytometric Assay
While ELISA and ACT Biotechnologies focus on measuring immune responses through antibody detection, cytometric assay assesses cellular-level immune reactions. These methods offer different perspectives on immune system reactivity, complementing each other within the LRA Comprehensive with Medications 349 Panel.
The Future of Medical Testing: ELISA / ACT Biotechnologies and Cytometric Assay
Emerging Trends in ELISA / ACT Biotechnologies
ELISA and ACT Biotechnologies continue to evolve with advancements in technology. Emerging trends include the development of multiplex assays, enabling simultaneous analysis of multiple antigens and antibodies. These innovations aim to enhance the efficiency and accuracy of diagnostic testing.
Innovations in Cytometric Assay
Similarly, cytometric assay is also experiencing advancements, such as the integration of high-speed flow cytometry and novel fluorochromes for improved resolution and sensitivity. These innovations offer greater precision in characterizing immune cell populations and further contribute to the diagnostic potential of the LRA Comprehensive with Medications 349 Panel.
The Impact of Technological Advancements on ELISA / ACT Biotechnologies and Cytometric Assay
Technological advancements in both ELISA / ACT Biotechnologies and cytometric assay have the potential to revolutionize medical testing. These improvements enable more accurate and comprehensive assessment of immune system reactivity, leading to better diagnosis and treatment strategies for patients with delayed hypersensitivity reactions.
In conclusion, the LRA Comprehensive with Medications 349 Panel by ELISA / ACT Biotechnologies and Cytometric Assay provides a comprehensive approach to assess delayed hypersensitivity reactions. While ELISA / ACT Biotechnologies focus on measuring immune responses through antibody detection, cytometric assay analyzes immune reactions at a cellular level. These methods offer valuable insights into the patient's immune system reactivity, complementing each other within the panel. As technology continues to advance, the future of medical testing looks promising, with innovations enhancing the diagnostic potential of both ELISA / ACT Biotechnologies and cytometric assay.