LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test)
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test)
LRA Additives/Preservatives Block 45 is a crucial component in the field of biotechnological testing. In this article, we will delve into the intricacies of LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies and compare it to the T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test) method. Through an in-depth exploration, we aim to shed light on the important role these two methodologies play in the analysis of additives and preservatives, and highlight their respective strengths and limitations.
Understanding LRA Additives/Preservatives Block 45
What are LRA Additives/Preservatives Block 45?
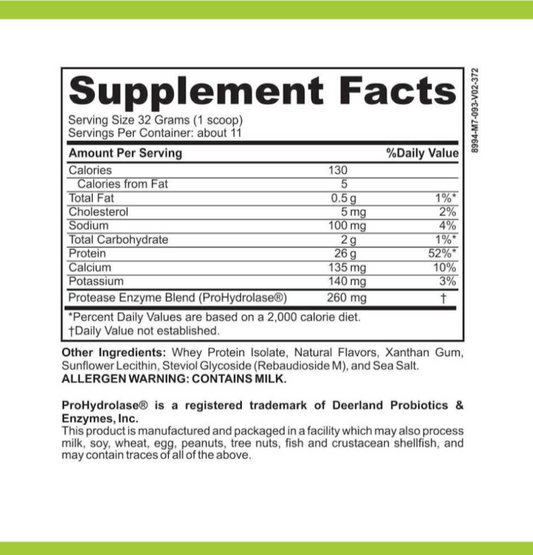
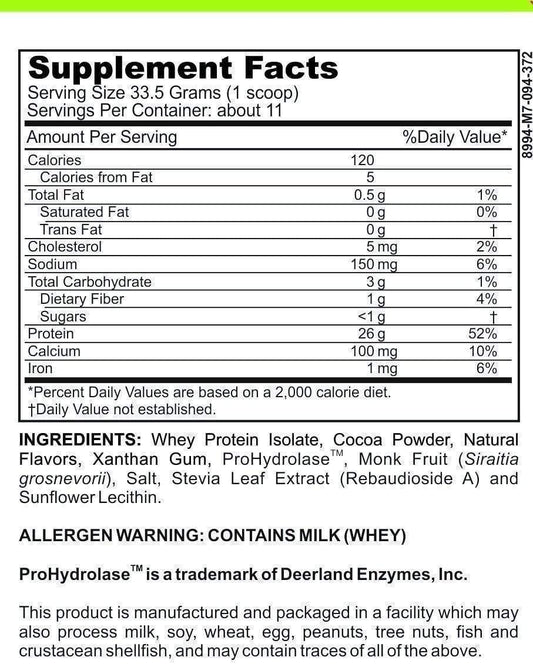
LRA Additives/Preservatives Block 45 refers to a set of substances commonly used in various industries for different purposes. These additives and preservatives are known to enhance the shelf life, appearance, flavor, and texture of food and other consumer products. The Block 45 variant is especially important as it encompasses a specific group of additives and preservatives subject to rigorous analysis.
When it comes to food production, LRA Additives/Preservatives Block 45 plays a vital role in ensuring that products reach consumers in optimal condition. These additives and preservatives act as powerful tools in preventing spoilage, maintaining freshness, and extending the overall lifespan of food items. From baked goods to dairy products, beverages to canned goods, the inclusion of Block 45 additives and preservatives has become a common practice in the food industry.
Not only do these additives and preservatives enhance the visual appeal of food, but they also contribute to the overall taste and texture. Block 45 additives are carefully selected to enhance flavors, add depth to aromas, and create a more enjoyable sensory experience for consumers. Additionally, they help maintain the desired texture of various food products, whether it's the smoothness of a sauce or the crispiness of a snack.
The Role of LRA Additives/Preservatives in Biotechnology
In the realm of biotechnology, additives and preservatives have far-reaching implications. They serve as stabilizers in pharmaceutical and medical preparations, ensuring the longevity and integrity of these products. Furthermore, they play a key role in the development of diagnostic tests, facilitating accurate and reliable results. LRA Additives/Preservatives Block 45, as a subset of these components, forms the cornerstone of targeted analysis.
Within the biotechnology field, the use of Block 45 additives and preservatives is critical for maintaining the stability and effectiveness of pharmaceutical formulations. These substances help prevent the degradation of active ingredients, ensuring that medications retain their potency over extended periods. By incorporating Block 45 additives and preservatives, pharmaceutical companies can confidently deliver medications that remain safe and effective throughout their shelf life.
Moreover, in the development of diagnostic tests, LRA Additives/Preservatives Block 45 plays a significant role in ensuring accurate and reliable results. These additives and preservatives help stabilize reagents, control reaction conditions, and minimize interference, thereby improving the precision and sensitivity of diagnostic assays. The inclusion of Block 45 additives and preservatives in diagnostic tests is crucial for obtaining consistent and trustworthy outcomes, which are vital for accurate disease detection and monitoring.
It is important to note that the analysis and regulation of LRA Additives/Preservatives Block 45 are subject to stringent standards and guidelines. Extensive research and testing are conducted to ensure that these additives and preservatives are safe for consumption and meet the necessary quality standards. Regulatory bodies closely monitor the use of Block 45 additives and preservatives to safeguard public health and maintain industry integrity.
The ELISA / ACT Biotechnologies Approach
Introduction to ELISA / ACT Biotechnologies
ELISA, or Enzyme-Linked Immunosorbent Assay, is a widely employed biochemical technique utilized in various scientific disciplines. It has revolutionized the field of diagnostics and research, allowing scientists to detect and quantify specific substances with high precision and accuracy. ELISA / ACT Biotechnologies, a leading biotech company, harnesses the principles of this method to identify and quantify LRA Additives/Preservatives Block 45 in samples, making significant contributions to the analysis of additives and preservatives.
With their expertise and cutting-edge technology, ELISA / ACT Biotechnologies has become pioneers in the field of biochemical analysis. Their team of highly skilled scientists and researchers work tirelessly to develop innovative approaches and solutions to address the challenges associated with identifying and measuring LRA Additives/Preservatives Block 45.
How ELISA / ACT Biotechnologies Utilizes LRA Additives/Preservatives Block 45
The ELISA / ACT Biotechnologies team employs a strategic approach to detect and measure LRA Additives/Preservatives Block 45. By utilizing specific antibodies designed to bind with these substances, they are able to identify their presence in a given sample. This process involves a series of carefully designed steps that ensure accurate and reliable results.
First, the sample is prepared by extracting and isolating the target substances. This step requires meticulous attention to detail to avoid any contamination or loss of the LRA Additives/Preservatives Block 45. Once the sample is ready, it is mixed with the specific antibodies that have been developed and optimized by the ELISA / ACT Biotechnologies team. These antibodies have a high affinity for the target substances, allowing them to bind specifically to LRA Additives/Preservatives Block 45.
After the antibodies have bound to the target substances, the sample is washed to remove any unbound antibodies or other interfering substances. This step is crucial to ensure the accuracy of the results, as any remaining unbound antibodies could lead to false positives or inaccurate measurements.
Finally, a detection system is employed to quantify the amount of LRA Additives/Preservatives Block 45 present in the sample. This system can vary depending on the specific requirements of the analysis, but commonly involves the use of enzymes or fluorescent markers that produce a measurable signal when they come into contact with the bound antibodies.
Through quantification techniques, ELISA / ACT Biotechnologies provides valuable insights into the concentration and distribution of LRA Additives/Preservatives Block 45. This information is crucial for various industries, including food and beverage, pharmaceuticals, and cosmetics, as it allows them to ensure the safety and quality of their products.
In addition to their expertise in LRA Additives/Preservatives Block 45 analysis, ELISA / ACT Biotechnologies also offers a wide range of other ELISA-based services. These include the detection and measurement of various allergens, pathogens, and biomarkers, making them a trusted partner for many research and diagnostic laboratories worldwide.
The T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test) Method
An Overview of the T.R.U.E. Test
The T.R.U.E. Test method, also known as Thin-layer Rapid Use Epicutaneous Test, is a dermatological technique designed to identify contact allergens. This test is particularly efficient when it comes to pinpointing skin sensitivities triggered by additives and preservatives, including those encompassed in LRA Additives/Preservatives Block 45.
The T.R.U.E. Test method revolutionized the field of dermatology by providing a quick and reliable way to diagnose contact allergies. Developed by renowned dermatologists, this method has become an invaluable tool for healthcare professionals worldwide. With its ability to identify specific allergens, it has helped countless patients find relief from skin irritations and discomfort.
One of the key advantages of the T.R.U.E. Test method is its non-invasive nature. Unlike other allergy tests that require blood samples or injections, the T.R.U.E. Test simply involves applying a thin layer of substances on the patient's skin. This makes it a preferred choice for individuals who may be hesitant or anxious about more invasive procedures.
The Application of LRA Additives/Preservatives in the T.R.U.E. Test
Within the T.R.U.E. Test method, LRA Additives/Preservatives Block 45 plays a crucial role as a comprehensive panel of substances for testing. By applying these additives and preservatives to a thin layer on the patient's skin, the dermatologist can discern if any allergic reactions occur due to their presence. This serves as a valuable diagnostic tool in identifying potential sensitivities.
LRA Additives/Preservatives Block 45 includes a wide range of common additives and preservatives that are frequently found in various products, such as cosmetics, personal care items, and household cleaners. These substances are known to cause allergic reactions in certain individuals, and the T.R.U.E. Test helps identify whether a patient has developed a sensitivity to any of them.
During the application of LRA Additives/Preservatives Block 45, the dermatologist carefully selects and prepares the substances to be tested. Each substance is applied to a separate patch on the patient's back, ensuring that they do not mix or interfere with each other. This meticulous process guarantees accurate results and minimizes the risk of false positives or false negatives.
Once the substances are applied, the patient is closely monitored for any signs of an allergic reaction. The dermatologist examines the skin for redness, swelling, or any other visible signs of irritation. In some cases, the patient may experience mild discomfort or itching, which is a normal response to the test. However, if a severe reaction occurs, immediate medical attention is provided to ensure the patient's safety and well-being.
After a specified period, typically 48 hours, the patches are removed, and the dermatologist carefully evaluates the test results. Any positive reactions, indicating a sensitivity to a particular substance, are documented and discussed with the patient. This information is crucial for the patient's future avoidance of potential allergens, allowing them to make informed choices about the products they use.
In conclusion, the T.R.U.E. Test method, coupled with the application of LRA Additives/Preservatives Block 45, provides dermatologists with an effective means of identifying contact allergens. By expanding our understanding of skin sensitivities triggered by additives and preservatives, this method has paved the way for improved patient care and management of allergic reactions. As research continues to advance, it is likely that the T.R.U.E. Test method will continue to evolve, offering even more precise and personalized diagnostic capabilities in the field of dermatology.
Comparative Analysis: ELISA / ACT Biotechnologies Vs T.R.U.E. Test
Methodology Comparison
When comparing ELISA / ACT Biotechnologies and the T.R.U.E. Test, it is important to recognize the fundamental differences in their methodologies. ELISA / ACT Biotechnologies employs a biochemical approach, whereas the T.R.U.E. Test is primarily a dermatological technique. The former focuses on quantification, while the latter aims to identify and diagnose allergic reactions.
Efficiency and Accuracy: A Comparative Study
In terms of efficiency and accuracy, both methodologies have their strengths. ELISA / ACT Biotechnologies' quantitative analysis provides precise measurements of LRA Additives/Preservatives Block 45 concentration. On the other hand, the T.R.U.E. Test allows for direct observation of skin reactions, making it an effective diagnostic tool for identifying allergies.
Pros and Cons: ELISA / ACT Biotechnologies Vs T.R.U.E. Test
ELISA / ACT Biotechnologies' strengths lie in its ability to analyze a wide range of samples and detect minute concentrations of LRA Additives/Preservatives Block 45. However, it requires specialized equipment and expertise. The T.R.U.E. Test, though limited in scope, offers a non-invasive approach for diagnosing sensitivities, but it may not identify specific allergenic compounds.
Future Perspectives and Developments
Potential Improvements in LRA Additives/Preservatives Block 45 Usage
As research progresses, there is an opportunity for further improvement in the applications of LRA Additives/Preservatives Block 45. Enhanced analysis techniques may enable quicker and more accurate identification and quantification of these additives and preservatives, leading to safer consumer products and greater efficiency in pharmaceutical preparations.
Future Trends in Biotechnological Testing Methods
The future of biotechnological testing methods shows promise for groundbreaking advancements. Scientists are continuously exploring innovative techniques that offer rapid and accurate results. These methods may encompass a wider range of additives and preservatives, furthering our understanding of their impacts on human health and well-being.
The Future of ELISA / ACT Biotechnologies and T.R.U.E. Test
Looking ahead, ELISA / ACT Biotechnologies continues to pave the way in biochemical analysis, constantly refining and expanding their methodologies. Meanwhile, the T.R.U.E. Test method is likely to evolve, incorporating emerging technologies and addressing limitations to enhance its diagnostic abilities.
In conclusion, the analysis of LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies and the T.R.U.E. Test method play pivotal roles in understanding the impact of additives and preservatives on human health and safety. While each methodology has its own strengths and limitations, their collective contribution to biotechnological testing cannot be understated. As research progresses, advancements in these fields hold the potential for a more comprehensive understanding of additives and preservatives, leading to improved consumer products and diagnostics.