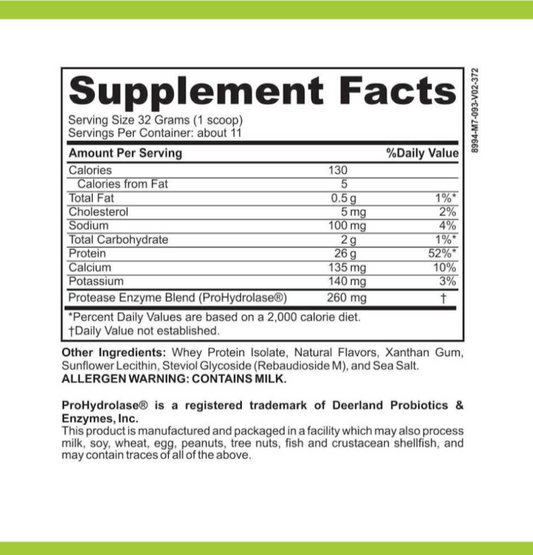
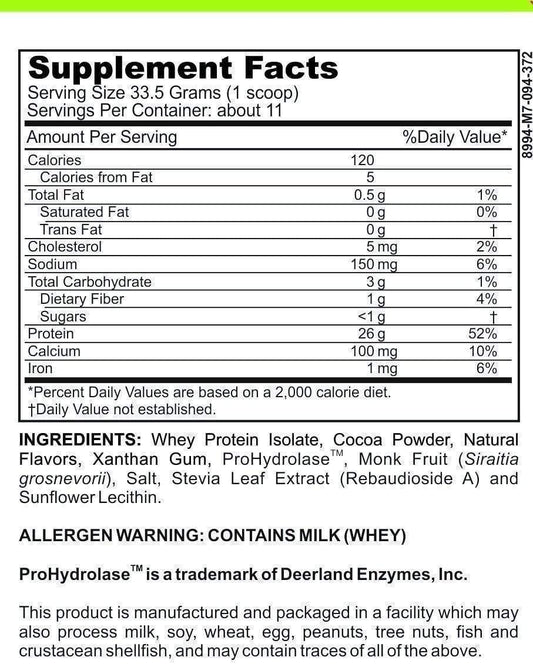
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs LEAPs Test
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs LEAPs Test
In recent years, the field of biotechnology has witnessed significant advancements in various testing methods. One such advancement is the LRA Additives/Preservatives Block 45, which can be analyzed using ELISA and ACT Biotechnologies. In this article, we will delve deeper into the understanding of LRA additives and preservatives and explore the role of ELISA and ACT Biotechnologies in this context. Additionally, we will discuss the LEAPs test, comparing it with ELISA and ACT, and speculate on the future of biotechnology testing. Let's begin!
Understanding LRA Additives/Preservatives Block 45
LRA additives, or Low Responder Antigens, are substances commonly found in food, cosmetics, and various pharmaceutical products. These additives, while generally considered safe for consumption, can trigger adverse reactions in individuals who are sensitive or allergic to them. Preservatives Block 45, on the other hand, are compounds used to enhance the shelf life and stability of products.
The use of LRA additives and preservatives has become increasingly prevalent in the biotechnology industry, raising concerns about their potential impact on human health. It is crucial to understand the function and effects of these substances to ensure the safety of the products we consume.
The Role of LRA Additives in Biotechnology
LRA additives play a significant role in the biotechnology industry. They are used to improve the stability, texture, flavor, and appearance of various products. Additionally, they can extend the shelf life of perishable items, reducing waste and minimizing the risk of contamination.
For example, in the food industry, LRA additives are commonly used to enhance the taste and appearance of processed foods. They can improve the texture of baked goods, making them moist and soft. Additionally, LRA additives are often used in the production of beverages to enhance their flavor profiles, making them more appealing to consumers.
Moreover, in the cosmetics industry, LRA additives are used to improve the texture and efficacy of skincare products. They can provide a smooth and luxurious feel to lotions and creams, making them more enjoyable to use. LRA additives can also enhance the fragrance of perfumes and colognes, creating a pleasant sensory experience for the users.
However, it is essential to note that certain individuals may exhibit adverse reactions to LRA additives, such as skin irritations, respiratory issues, digestive problems, and allergic reactions. These reactions can range from mild to severe, and it is crucial to identify and address them effectively.
Understanding the Function of Preservatives Block 45
Preservatives Block 45, as the name suggests, are substances used to block the growth of microorganisms and prevent spoilage of products. These preservatives inhibit the proliferation of bacteria, yeast, and molds that can potentially contaminate and degrade the quality of various perishable goods.
Preservatives Block 45 not only ensure the safety of the products but also enhance their overall quality and longevity. They are widely used in the food and beverage industry, as well as in pharmaceuticals and personal care products.
In the food industry, Preservatives Block 45 are commonly used in processed and packaged foods to extend their shelf life. By inhibiting the growth of bacteria and other microorganisms, these preservatives help prevent spoilage and maintain the freshness of the products for a longer period. This not only reduces food waste but also allows consumers to enjoy the products for an extended period.
Furthermore, in the pharmaceutical industry, Preservatives Block 45 are used in various medications to ensure their stability and efficacy. These preservatives help prevent the growth of harmful bacteria and maintain the integrity of the medications, ensuring that they remain safe and effective for use.
Similarly, in the personal care industry, Preservatives Block 45 are used in products such as shampoos, lotions, and creams to prevent the growth of bacteria and fungi. This helps maintain the quality and safety of these products, ensuring that they remain free from contamination and safe for use.
Overall, the use of Preservatives Block 45 is crucial in various industries to ensure the safety, quality, and longevity of products. By inhibiting the growth of microorganisms, these preservatives help protect consumers from potential health risks and ensure that the products maintain their desired attributes over time.
An Overview of ELISA / ACT Biotechnologies
ELISA, or Enzyme-Linked Immunosorbent Assay, is a widely used biotechnology testing method that enables the detection and quantification of specific substances in samples. It involves the use of specific antibodies that can bind to the target substances and produce measurable signals.
ACT Biotechnologies, on the other hand, specializes in advanced cellular technology that enables the analysis of cell function and response to different stimuli. This methodology provides valuable insights into the physiological effects of various substances, including LRA additives and preservatives.
The Science Behind ELISA
ELISA employs a series of specific steps to detect and measure the presence of LRA additives and preservatives. These steps include sample preparation, antigen immobilization, antibody binding, signal amplification, and detection. By quantifying the signals generated during these processes, ELISA can accurately determine the concentration of the target substances.
During the sample preparation stage, the sample is carefully collected and processed to ensure accurate results. This may involve various techniques such as centrifugation, filtration, or extraction. The quality of the sample is crucial for obtaining reliable data.
Once the sample is prepared, the antigen immobilization step takes place. The target substances are immobilized onto a solid surface, such as a microplate, to facilitate their interaction with the antibodies. This immobilization ensures that only the desired substances are detected and measured.
The next step involves the binding of specific antibodies to the immobilized target substances. These antibodies are designed to recognize and bind to the target substances with high affinity and specificity. This binding event forms the basis of signal generation in ELISA.
Signal amplification is an essential step in ELISA. It enhances the detectability of the target substances by increasing the intensity of the generated signals. Various amplification strategies can be employed, such as the use of enzyme-conjugated antibodies or signal-enhancing molecules.
Finally, the detection step involves measuring the signals generated during the previous steps. This can be done using various techniques, such as colorimetric, fluorometric, or luminescent methods. The intensity of the signals is proportional to the concentration of the target substances, allowing for accurate quantification.
The sensitivity and specificity of ELISA make it a powerful tool for assessing the presence and levels of LRA additives and preservatives in various products, aiding in quality control and regulatory compliance.
The Impact of ACT Biotechnologies in the Industry
ACT Biotechnologies complements ELISA by providing a deeper understanding of how LRA additives and preservatives interact with living organisms. By analyzing the cellular response to these substances, ACT can reveal potential physiological effects and assist in risk assessment.
ACT Biotechnologies utilizes advanced cellular technology to study the effects of LRA additives and preservatives on different cell types. This technology allows researchers to observe and measure changes in cell morphology, viability, proliferation, and gene expression in response to these substances.
Furthermore, ACT Biotechnologies can investigate the mechanisms of action of LRA additives and preservatives at the cellular level. This knowledge is crucial for understanding how these substances may affect various physiological processes and potentially contribute to health-related issues.
The insights gained from ACT Biotechnologies can be crucial in refining the use of LRA additives and preservatives, ensuring their safety, and minimizing risks associated with their consumption. By understanding the cellular response to these substances, manufacturers and regulatory bodies can make informed decisions regarding their use in different products.
ACT Biotechnologies' expertise in cellular technology also extends to other areas of biotechnology research. Their methodologies can be applied to study the effects of various compounds, drugs, or environmental factors on cell function and response. This broadens the scope of their impact in the industry and contributes to advancements in biomedical research.
The LEAPs Test: What You Need to Know
The LEAPs test, or Ligand Epitope Antigen Presentation System test, is another method used to assess the reactivity of the immune system to specific substances. This test focuses on identifying and measuring the immune response triggered by LRA additives and preservatives in individuals.
The Importance of the LEAPs Test
The LEAPs test plays a vital role in identifying individuals who may exhibit adverse reactions to LRA additives and preservatives. By measuring immune responses, it provides valuable information for diagnosing sensitivities and formulating appropriate prevention and treatment strategies.
How the LEAPs Test Works
The LEAPs test involves exposing a patient's blood sample to various LRA additives and preservatives. The immune system's response is then measured by assessing changes in immune cell activity, cytokine production, and other immunological markers.
This comprehensive approach helps healthcare professionals identify potential triggers, customize diets, and recommend suitable alternatives for individuals with sensitivities or allergies.
Comparing ELISA / ACT Biotechnologies and LEAPs Test
Key Differences Between ELISA / ACT Biotechnologies and LEAPs Test
While ELISA and ACT Biotechnologies focus on analyzing and quantifying the presence of LRA additives and preservatives in products, the LEAPs test assesses the immune reactivity of individuals. These methods serve different purposes but can complement each other in ensuring product safety and identifying susceptible individuals.
Understanding the Advantages and Disadvantages of Each Method
ELISA and ACT Biotechnologies offer high sensitivity and specificity in detecting LRA additives and preservatives, aiding in quality control and risk assessment. However, they may not provide direct information on individual reactions or sensitivities.
On the other hand, the LEAPs test is specifically designed to assess individual immune responses, providing personalized insights. However, it may not directly quantify the concentration of LRA additives and preservatives present in the tested samples.
The Future of Biotechnology: LRA Additives, ELISA, ACT, and LEAPs
As the field of biotechnology continues to advance, significant developments are expected in LRA testing methods and their applications. Predicted developments include improved sensitivity and specificity, automation, and a more comprehensive understanding of the physiological effects and sensitivities associated with LRA additives and preservatives.
Predicted Developments in Biotechnology Testing
In the future, we can anticipate the emergence of novel testing methodologies that combine the strengths of ELISA, ACT Biotechnologies, and the LEAPs test. These integrated approaches may provide a more holistic understanding of LRA additives and preservatives, their impact on human health, and personalized recommendations for individuals.
The Potential Impact of These Technologies on the Biotechnology Industry
With ongoing advancements, the biotechnology industry will benefit from enhanced quality control, faster product development cycles, and improved risk assessments. Furthermore, by catering to individual sensitivities and allergies, companies can establish themselves as leaders in consumer safety and offer more personalized products.
In conclusion, LRA additives and preservatives play a crucial role in various industries, but their potential impact on human health necessitates robust testing methods such as ELISA, ACT Biotechnologies, and the LEAPs test. These methodologies offer valuable insights into the presence, concentration, and immune reactivity associated with LRA additives and preservatives.
As the field continues to evolve, we can anticipate exciting developments that will further enhance our understanding and enable more personalized approaches to mitigate risks and optimize product safety. With the future advancements in biotechnology testing, consumers can expect a safer and more tailored experience with the products they consume.