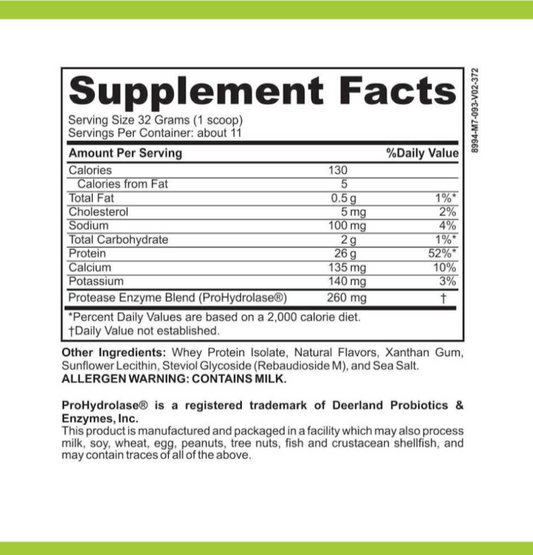
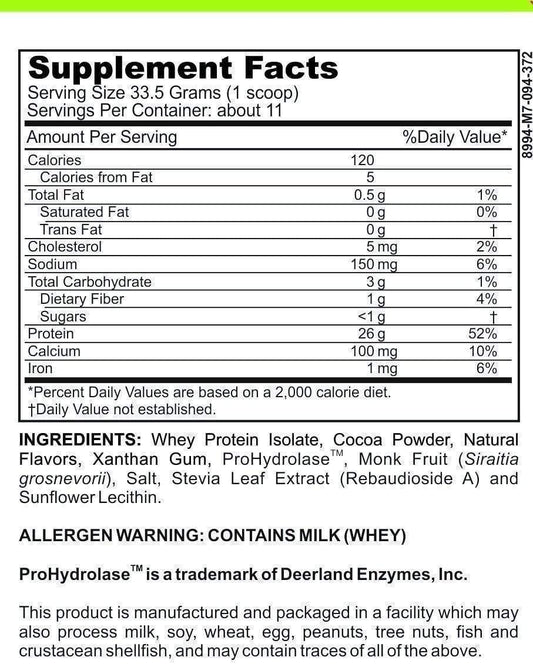
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs IgG Test
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs IgG Test
In the world of biotechnology and immunology, there are various testing methods available to identify and analyze specific substances within a given sample. One such method is the LRA Additives/Preservatives Block 45 test, conducted using ELISA/ACT Biotechnologies, which aims to detect the presence and effects of additives and preservatives in biological materials. This article will delve into the intricacies of this testing method, its key features, and compare it with the widely-used IgG Test.
Understanding LRA Additives/Preservatives Block 45
The LRA Additives/Preservatives Block 45 is a testing approach that allows researchers and healthcare professionals to uncover potential sensitivities or intolerance to additives and preservatives commonly found in food, medications, and other products. By utilizing ELISA/ACT Biotechnologies, this test aims to provide an in-depth understanding of the individual's immune response to these substances.
The Role of LRA Additives in Biotechnology
Additives play a crucial role in biotechnology, as they enhance the stability, shelf life, and effectiveness of various products. They are carefully selected and incorporated into formulations to ensure optimal results. However, it is important to note that while these additives are generally considered safe, certain individuals may exhibit adverse reactions to them. These reactions can range from mild to severe, and may include symptoms such as skin rashes, digestive issues, or respiratory problems.
The LRA Additives/Preservatives Block 45 test helps identify these potential sensitivities, enabling individuals to make informed decisions about their dietary and medication choices. By understanding their specific immune response to these additives, individuals can avoid products that may trigger unwanted symptoms, leading to a better quality of life.
Key Features of Preservatives Block 45
One of the notable features of the LRA Additives/Preservatives Block 45 test is its comprehensive coverage of 45 commonly encountered additives and preservatives. This wide range of substances enables researchers and healthcare professionals to obtain a comprehensive understanding of an individual's potential sensitivities. It is crucial to test for a broad spectrum of additives and preservatives, as different individuals may react differently to various substances.
By detecting even minor immune responses, this test allows for precise identification of problematic substances. This level of detail is essential in guiding individuals towards making informed choices about the products they consume or use. It empowers them to avoid potential allergens or irritants that may negatively impact their health and well-being.
The sensitivity and accuracy of the LRA Additives/Preservatives Block 45 test are further enhanced through the use of ELISA/ACT Biotechnologies. This cutting-edge technology employs enzyme-linked immunosorbent assay (ELISA) principles to precisely detect and measure specific antibodies associated with immune reactions. ELISA is a widely recognized and trusted method in the field of immunology, known for its reliability and precision.
ACT Biotechnologies, on the other hand, utilizes advanced techniques to amplify the signals generated by ELISA, allowing for even more precise and accurate measurements. This combination of ELISA and ACT Biotechnologies creates a powerful testing method for additives and preservatives, providing researchers and healthcare professionals with valuable insights into an individual's immune response.
Overall, the LRA Additives/Preservatives Block 45 test is a valuable tool in identifying and understanding potential sensitivities to additives and preservatives. By utilizing state-of-the-art technology and comprehensive coverage of substances, it enables individuals to take control of their health and make informed choices about the products they consume.
Deep Dive into ELISA / ACT Biotechnologies
ELISA/ACT Biotechnologies is a cutting-edge technology used in various fields, including medical research, diagnostics, and biopharmaceutical development. It serves as an invaluable tool in identifying and quantifying specific substances of interest within complex biological samples. Understanding the science behind this technology can shed light on its role in the LRA Additives/Preservatives Block 45 test.
The Science Behind ELISA Technology
ELISA technology utilizes the binding properties of antibodies to detect and measure the presence of a target substance in a sample. The process involves immobilizing antigens (substances of interest) on a solid surface, such as a microplate, and introducing the sample containing antibodies that can bind to these antigens. Through specific reaction steps, the presence and concentration of the target substance can be determined.
One of the key steps in ELISA technology is the immobilization of antigens on a solid surface. This can be achieved through various methods, such as physical adsorption, covalent binding, or affinity capture. The choice of immobilization method depends on the nature of the antigens and the desired sensitivity of the assay.
Once the antigens are immobilized, the sample containing antibodies is added. These antibodies are specifically designed to bind to the target substance, forming an antigen-antibody complex. This complex can be detected through various means, such as enzyme-conjugated secondary antibodies or fluorescently labeled antibodies.
ELISA technology is highly sensitive, allowing for the detection of even trace amounts of a substance. This sensitivity is achieved through signal amplification methods, such as enzyme-substrate reactions or fluorescence enhancement. These amplification strategies enable researchers to accurately quantify the concentration of the target substance in the sample.
Furthermore, ELISA technology offers different formats, including direct, indirect, sandwich, and competitive ELISA, each with its own advantages and applications. These formats allow for flexibility in experimental design and cater to the specific needs of different assays.
ELISA technology's versatility and reliability have made it indispensable in various fields, including biotechnological testing like the LRA Additives/Preservatives Block 45 test. Its ability to accurately measure the presence and concentration of target substances in complex samples has revolutionized the way researchers approach diagnostics and biopharmaceutical development.
Exploring the Applications of ACT Biotechnologies
ACT Biotechnologies is an integral part of the ELISA/ACT system used in the LRA Additives/Preservatives Block 45 test. This component provides a standardized method for sample preparation and the subsequent immunological reactions required for accurate results. ACT Biotechnologies ensures consistency, reliability, and efficiency throughout the testing process.
ACT Biotechnologies plays a crucial role in sample preparation, ensuring that the samples are properly handled and processed before the ELISA assay. This includes steps such as sample dilution, centrifugation, and filtration to remove any interfering substances that could affect the accuracy of the results.
Furthermore, ACT Biotechnologies provides the necessary reagents and controls for the immunological reactions in the ELISA assay. These reagents are carefully formulated to ensure optimal performance and reproducibility. Quality control measures are implemented to guarantee the reliability and accuracy of the results.
Beyond the LRA Additives/Preservatives Block 45 test, ACT Biotechnologies finds applications in various fields, such as clinical diagnostics, pharmaceutical development, and research quality control. Its compatibility with ELISA technology makes it a versatile tool for accurately measuring immune responses and understanding the implications of exposure to various substances.
In clinical diagnostics, ACT Biotechnologies can be used to detect and quantify specific biomarkers associated with diseases or conditions. This information can aid in early detection, monitoring treatment effectiveness, and predicting patient outcomes.
Pharmaceutical companies can utilize ACT Biotechnologies to assess the immunogenicity of drug candidates, ensuring their safety and efficacy. By measuring the immune response to these candidates, researchers can identify potential adverse reactions and make informed decisions during drug development.
Research quality control is another area where ACT Biotechnologies excels. It allows researchers to validate their experimental protocols, ensuring that the results obtained are accurate and reliable. By using standardized methods and controls provided by ACT Biotechnologies, researchers can confidently interpret their findings and contribute to the advancement of scientific knowledge.
The IgG Test: An Overview
While the LRA Additives/Preservatives Block 45 test provides insights into sensitivities related to additives and preservatives, the IgG (immunoglobulin G) test focuses on identifying immune responses to specific antigens. As one of the major immunoglobulin classes, IgG plays a critical role in the body's immune defense mechanisms. Understanding the importance and functioning of the IgG test adds a broader perspective to biotechnological testing.
The Importance of the IgG Test in Immunology
The IgG test plays a pivotal role in immunological assessments, aiding in the identification of food sensitivities, allergies, and autoimmune conditions. By measuring the levels of specific IgG antibodies in a blood sample, this test helps determine an individual's immune response to a particular substance.
These findings equip healthcare professionals with valuable information to guide patients towards suitable dietary adjustments and potentially alleviate symptoms associated with immune reactions. The IgG test provides a personalized approach to understanding and managing immune-related conditions.
How the IgG Test Works
The IgG test involves analyzing a blood sample for the presence and quantity of IgG antibodies. The process begins with the collection of a blood sample, which is then analyzed in a laboratory setting. Using various techniques, such as ELISA or immunoblotting, specific IgG antibodies are identified and quantified.
Based on the results, healthcare professionals can determine the substances to which an individual may have developed immune tolerance, as well as those that trigger immune reactions. This information allows for tailored treatment plans and dietary modifications to enhance overall well-being.
Comparing ELISA / ACT Biotechnologies and IgG Test
While both the LRA Additives/Preservatives Block 45 test utilizing ELISA/ACT Biotechnologies and the IgG test provide valuable insights into an individual's immune response, there are certain differences in their methodology, effectiveness, and scope of analysis.
Similarities and Differences in Methodology
Both the LRA Additives/Preservatives Block 45 test and the IgG test rely on the principles of immunological reactions. They involve detecting specific antibodies in a sample and measuring their levels. However, the LRA Additives/Preservatives Block 45 test focuses on identifying immune responses to specific additives and preservatives, while the IgG test encompasses a broader range of substances.
Furthermore, the LRA Additives/Preservatives Block 45 test utilizes ELISA/ACT Biotechnologies, providing a comprehensive approach to detect and measure immune responses with high sensitivity. On the other hand, the IgG test can employ different techniques, such as ELISA or immunoblotting, to assess IgG antibody levels.
Effectiveness and Accuracy: A Comparative Analysis
The effectiveness and accuracy of both testing methods depend on various factors, including the specific substances analyzed and the individual's immune system. The LRA Additives/Preservatives Block 45 test enables focused insights into potential sensitivities related to additives and preservatives, allowing for targeted dietary modifications.
The broader scope of the IgG test provides a comprehensive understanding of an individual's immune response to various antigens. This information is particularly valuable for managing conditions, such as food sensitivities or autoimmune disorders. However, the interpretation of IgG test results must consider individual variations and other clinical factors.
Future Trends in Biotechnological Testing
The field of biotechnological testing continues to advance, driven by technological developments, evolving research, and the expanding understanding of the human immune system. The future holds promising possibilities for the LRA Additives/Preservatives Block 45 test based on ELISA/ACT Biotechnologies and the IgG test.
The Potential of ELISA / ACT Biotechnologies
ELISA/ACT Biotechnologies, with its remarkable sensitivity and accurate measurement capabilities, may enable further expansion of the LRA Additives/Preservatives Block 45 test. As research uncovers additional additives and preservatives affecting immune responses, the inclusion of these substances in testing panels could refine diagnostic approaches and provide more comprehensive insights.
The Evolving Role of IgG Testing in Modern Medicine
IgG testing continues to be an essential tool in immunology and personalized healthcare. With ongoing advancements in technology and scientific understanding, the scope of substances analyzed and the accuracy of IgG tests are expected to improve. Furthermore, the integration of omics technologies, such as proteomics and genomics, may further enhance the capability to assess an individual's immune response and revolutionize personalized medicine.
In conclusion, the LRA Additives/Preservatives Block 45 test conducted using ELISA/ACT Biotechnologies and the IgG test are valuable tools in biotechnological testing. While the former allows for targeted identification of sensitivities to additives and preservatives, the latter provides a broader perspective on immune responses. The future of biotechnological testing holds exciting potential for advancements in understanding and managing immune-related conditions, leading to improved healthcare outcomes for individuals.