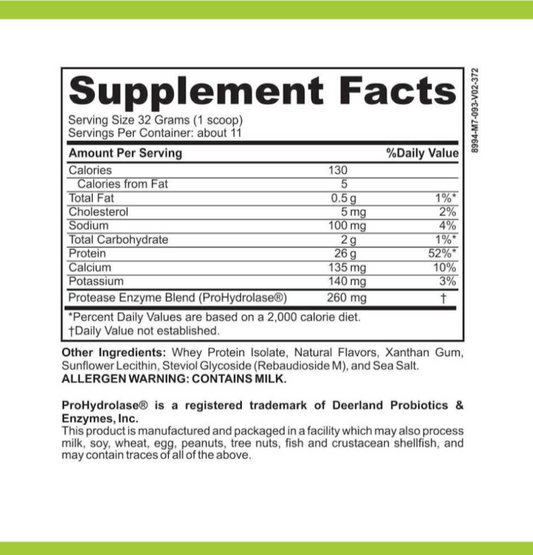
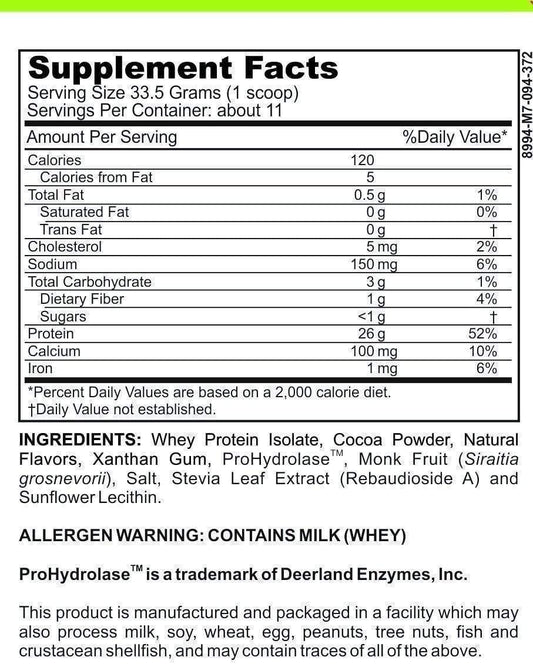
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs Double Blind Placebo Controlled Food Challenges
LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies Vs Double Blind Placebo Controlled Food Challenges
In recent years, concerns about food allergies and sensitivities have become increasingly prevalent. Many people experience adverse reactions to certain food additives and preservatives, which can significantly impact their quality of life. To address these concerns, two diagnostic methods have emerged as potential solutions: LRA Additives/Preservatives Block 45 by ELISA / ACT Biotechnologies and Double Blind Placebo Controlled Food Challenges. In this article, we will explore the intricacies of these methods, their scientific foundations, and their respective strengths and limitations.
Understanding LRA Additives/Preservatives Block 45
If you've ever wondered what exactly LRA Additives/Preservatives Block 45 is, you're not alone. LRA Additives/Preservatives Block 45 is a comprehensive test designed to identify and measure individual sensitivities to various food additives and preservatives. It is based on the concept of LRA, which stands for lymphocyte response assay, a specialized laboratory test that measures immune responses to specific substances.
Food additives and preservatives are substances that are added to processed foods to improve their taste, texture, and shelf life. While these additives are generally recognized as safe by regulatory bodies, some individuals may have adverse reactions to them, resulting in symptoms such as gastrointestinal issues, skin disorders, or respiratory problems.
The LRA Additives/Preservatives Block 45 test aims to provide individuals with a deeper understanding of their body's response to these additives and preservatives. By expanding our knowledge in this area, we can make more informed decisions about the foods we consume, ensuring our overall well-being.
What are LRA Additives/Preservatives Block 45?
LRA Additives/Preservatives Block 45 is a specific panel within the larger LRA test, focusing specifically on food additives and preservatives. The panel consists of 45 commonly used additives and preservatives, including but not limited to artificial colors, flavors, sweeteners, and preservatives.
Utilizing ELISA (enzyme-linked immunosorbent assay) technology, the LRA Additives/Preservatives Block 45 test aims to identify if an individual's immune system is reacting to any of these additives or preservatives. By measuring the levels of specific antibodies in the blood, the test can indicate sensitivities and potential triggers for adverse reactions.
Understanding the specific additives and preservatives that our bodies may react to is crucial in managing our health. LRA Additives/Preservatives Block 45 provides us with a detailed analysis, allowing us to take proactive steps in avoiding potential triggers and maintaining a healthy lifestyle.
The Role of LRA Additives/Preservatives in Food Industry
Food additives and preservatives play a crucial role in the food industry. They help to maintain food freshness, enhance taste and appearance, and extend shelf life. However, the growing prevalence of food allergies and sensitivities has raised concerns about the safety of these additives.
The LRA Additives/Preservatives Block 45 test seeks to address these concerns by providing individuals with valuable information about their specific sensitivities. Armed with this knowledge, they can make more informed choices about the foods they consume, minimizing the risk of adverse reactions and improving their overall well-being.
It is important for food manufacturers to consider the impact of additives and preservatives on consumer health. By being mindful of potential sensitivities and allergies, the industry can develop safer and more inclusive food options, catering to a wider range of dietary needs.
Furthermore, the LRA Additives/Preservatives Block 45 test can serve as a valuable tool for food manufacturers. By understanding the specific additives and preservatives that individuals may react to, they can create products that are not only delicious but also safe for a broader consumer base.
The Science Behind ELISA / ACT Biotechnologies
ELISA / ACT Biotechnologies is the laboratory that specializes in conducting the LRA Additives/Preservatives Block 45 test. To better understand their contribution, let's delve into the science behind this innovative approach to food sensitivity testing.
Introduction to ELISA / ACT Biotechnologies
ELISA / ACT Biotechnologies is a renowned scientific laboratory dedicated to advancing diagnostic technologies and improving healthcare outcomes. With their expertise in ELISA, a widely used immunological test, they have developed the LRA Additives/Preservatives Block 45 test to assess individual sensitivities to food additives and preservatives.
The laboratory is equipped with state-of-the-art facilities and staffed by a team of highly skilled scientists and technicians. They work tirelessly to ensure accurate and reliable test results, adhering to strict quality control measures and following standardized protocols.
ELISA / ACT Biotechnologies collaborates with leading experts in the field of immunology and food science to stay at the forefront of research and development. Their commitment to innovation and scientific excellence sets them apart in the industry.
How ELISA / ACT Biotechnologies Work with LRA Additives/Preservatives
When conducting the LRA Additives/Preservatives Block 45 test, ELISA / ACT Biotechnologies utilizes ELISA technology to measure the immune response to specific food additives and preservatives. This cutting-edge technique allows them to detect the presence of antibodies that recognize and react to these substances.
The ELISA (Enzyme-Linked Immunosorbent Assay) test is based on the principle of antigen-antibody interaction. In this case, the antigens are the food additives and preservatives, while the antibodies are the immune molecules produced by the body in response to these substances.
The blood sample provided by the individual is carefully processed to isolate the serum, which contains the antibodies of interest. This serum is then incubated with the additives and preservatives present in the LRA panel, enabling the laboratory to determine if there is an immune response.
During the incubation period, the antibodies in the serum bind to the specific additives and preservatives they recognize. This binding event triggers a series of enzymatic reactions, resulting in the production of a measurable signal.
ELISA / ACT Biotechnologies employs advanced detection systems to quantify this signal, allowing them to identify which additives or preservatives may be triggering adverse reactions. The levels of specific antibodies are carefully measured, providing valuable insights into an individual's sensitivities.
It is important to note that ELISA / ACT Biotechnologies ensures the accuracy of their results by using appropriate positive and negative controls, as well as conducting rigorous validation studies. This ensures that the test is reliable and reproducible, providing healthcare professionals with valuable information to guide their patients' dietary choices.
The LRA Additives/Preservatives Block 45 test offered by ELISA / ACT Biotechnologies is a powerful tool in the field of food sensitivity testing. By accurately assessing an individual's immune response to specific additives and preservatives, it allows for personalized dietary recommendations and improved management of adverse reactions.
Double Blind Placebo Controlled Food Challenges Explained
Double Blind Placebo Controlled Food Challenges are another approach commonly used to investigate adverse reactions to food additives and preservatives. Let's explore how these challenges work and their significance in diagnosing food sensitivities.
The Importance of Double Blind Placebo Controlled Food Challenges
Double Blind Placebo Controlled Food Challenges are considered the "gold standard" in diagnosing food allergies and sensitivities. These challenges involve administering a suspected food or substance, along with a placebo, in a controlled, double-blind manner, meaning neither the patient nor the administering healthcare professional knows which substance is being tested at any given time.
By employing this rigorous scientific method, any observed adverse reactions can be confidently attributed to the specific food additive or preservative being tested. This helps to eliminate potential biases and ensures accurate diagnosis and treatment plans.
The Process of Double Blind Placebo Controlled Food Challenges
The process of Double Blind Placebo Controlled Food Challenges involves multiple stages. Firstly, the patient is carefully assessed to determine their medical history, symptoms, and suspected triggers. Next, they undergo an initial screening to rule out any pre-existing allergies or contraindications.
During the challenge, the patient is given varying doses of the suspected food additive or preservative and a placebo, with strict controls in place to monitor their immediate and delayed reactions. The challenge is overseen by healthcare professionals who closely observe and record any symptoms or adverse reactions. This data is then used to evaluate the patient's sensitivity to the tested substance.
Comparing ELISA / ACT Biotechnologies and Double Blind Placebo Controlled Food Challenges
Both ELISA / ACT Biotechnologies and Double Blind Placebo Controlled Food Challenges serve as valuable tools in identifying food sensitivities. Let's examine the similarities and differences between these two approaches and consider their respective strengths and limitations.
Similarities and Differences
While both methods aim to identify adverse reactions to food additives and preservatives, they employ different mechanisms and approaches. ELISA / ACT Biotechnologies utilizes blood tests and laboratory analysis to detect immune responses, while Double Blind Placebo Controlled Food Challenges involve ingesting the suspected substances in a controlled environment.
One key similarity is that both methods provide valuable information regarding an individual's sensitivities. However, they differ in terms of cost, availability, and the potential for false positives and false negatives. Therefore, it is essential to consult with healthcare professionals to determine which method best suits an individual's needs.
Strengths and Limitations of Each Method
ELISA / ACT Biotechnologies offers a comprehensive and efficient way to identify specific sensitivities to food additives and preservatives. It provides a broad overview of potential triggers, allowing individuals to make informed dietary choices accordingly. However, it does not replicate real-life consumption scenarios and may not capture delayed reactions.
On the other hand, Double Blind Placebo Controlled Food Challenges offer a controlled environment that closely simulates real-world conditions. They allow for immediate identification of adverse reactions and are effective at capturing immediate responses. However, they require significant resources, oversight from medical professionals, and may not be suitable for individuals with severe allergic reactions.
Case Studies and Research Findings
Let's delve into some notable case studies and recent research findings regarding food sensitivities and the efficacy of the diagnostic methods discussed.
Notable Case Studies
In a case study conducted by researchers at a leading allergy clinic, a patient who experienced chronic gastrointestinal issues was diagnosed with a sensitivity to a common food additive through the LRA Additives/Preservatives Block 45 test. Armed with this knowledge, the patient was able to eliminate the trigger from their diet, resulting in a significant improvement in their symptoms.
In contrast, a study published in a respected medical journal compared the results of Double Blind Placebo Controlled Food Challenges with those of a comprehensive panel of blood tests, including ELISA-based assays. The study found close agreement between the two methods, reinforcing the effectiveness of both approaches in diagnosing food sensitivities.
Recent Research Findings
A recent study conducted by a team of researchers demonstrated the potential benefits of combining ELISA / ACT Biotechnologies and Double Blind Placebo Controlled Food Challenges. By using ELISA to identify potential triggers and subsequently conducting controlled challenges, healthcare professionals gained a comprehensive understanding of individual sensitivities, enabling them to craft personalized treatment plans.
Furthermore, ongoing research aims to improve the accuracy and accessibility of diagnostic methods for food sensitivities. Scientists are continually developing new tests, refining existing protocols, and exploring emerging technologies to enhance diagnostic capabilities and improve the lives of individuals affected by adverse reactions to food additives and preservatives.
Conclusion
As our understanding of food allergies and sensitivities grows, so do the methods available for diagnosis and management. The LRA Additives/Preservatives Block 45 test by ELISA / ACT Biotechnologies and Double Blind Placebo Controlled Food Challenges offer complementary approaches to identifying individual sensitivities.
While both methods have their respective strengths and limitations, they contribute significantly to the field of food sensitivity diagnostics. By leveraging the scientific foundations and expertise behind these methods, individuals can gain valuable insights into their unique sensitivities, empowering them to make informed decisions about their diet and improve their overall well-being.
As research continues to advance, it is our hope that further progress in diagnostic methods will enable more accurate and accessible identification of food sensitivities, ultimately leading to improved healthcare outcomes for all.