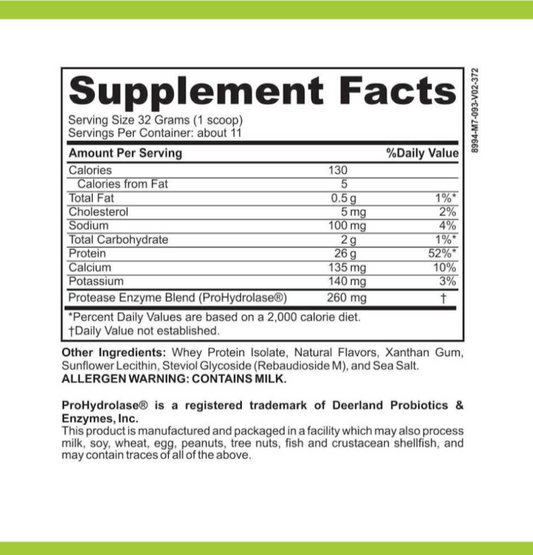
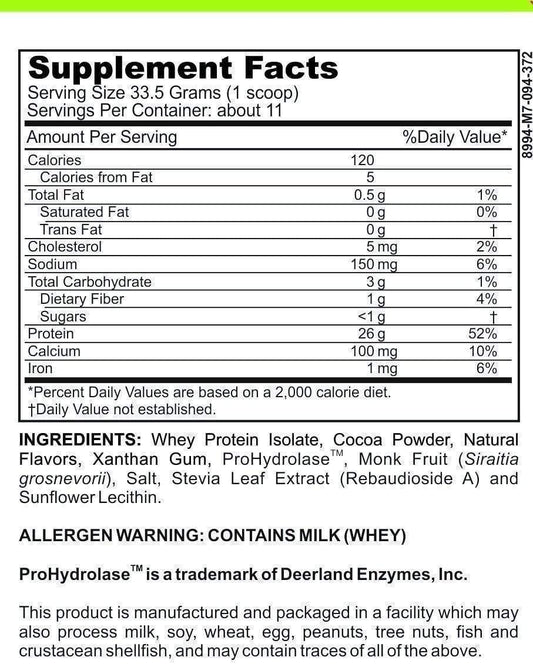
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs Vega Test
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs Vega Test
In today's world, where food allergies and intolerances are on the rise, it becomes increasingly important to understand the potential triggers that can affect our well-being. LRA Additives/Preservatives are substances commonly found in processed foods and beverages that have been linked to various health issues. In this article, we will explore the significance of LRA Additives/Preservatives Block 15 and compare the detection methods provided by ELISA / ACT Biotechnologies and the Vega Test.
Understanding LRA Additives/Preservatives Block 15
Before diving into the specifics of Block 15, let's understand what LRA Additives/Preservatives are in general.
LRA Additives/Preservatives are chemical substances that are added to food and beverages to enhance their shelf-life, appearance, taste, or texture. They play a crucial role in the food industry by preventing spoilage, maintaining freshness, and improving overall product quality. These additives can be found in a wide range of products, including processed foods, canned goods, baked goods, beverages, and even cosmetics.
These additives are carefully regulated by food authorities to ensure that they are safe for consumption. However, concerns have been raised about the potential adverse effects of these substances on human health, particularly in individuals who have developed sensitivities or allergies to certain additives.
What are LRA Additives/Preservatives?
LRA Additives/Preservatives are chemical substances that are added to food and beverages to enhance their shelf-life, appearance, taste, or texture. While they may seem harmless, these additives have raised concerns due to potential adverse reactions in certain individuals.
These additives can be categorized into different groups based on their function and chemical composition. Some common types of LRA Additives/Preservatives include antioxidants, antimicrobials, emulsifiers, flavor enhancers, and stabilizers. Each of these additives serves a specific purpose in food production and preservation.
Antioxidants, for example, help prevent the oxidation of fats and oils, extending the shelf-life of products and maintaining their quality. Antimicrobials, on the other hand, inhibit the growth of bacteria, yeasts, and molds, preventing spoilage and foodborne illnesses. Emulsifiers are used to stabilize mixtures of oil and water, ensuring a consistent texture and preventing separation. Flavor enhancers are added to intensify the taste and aroma of food, making it more appealing to consumers. Stabilizers help maintain the texture and consistency of products, preventing them from breaking down or becoming too runny.
While these additives serve important functions in the food industry, there have been concerns about their potential health effects. Some individuals may experience adverse reactions to certain additives, leading to symptoms such as headaches, digestive issues, skin rashes, or respiratory problems. These reactions can vary in severity, with some individuals experiencing mild discomfort while others may have more severe symptoms.
The Role of Block 15 in LRA Additives/Preservatives
Block 15 is a specific group of LRA Additives/Preservatives that has been identified as a potential trigger for adverse reactions. These reactions can range from mild discomfort to more severe symptoms in individuals who have developed sensitivities or allergies to these substances.
Block 15 additives are commonly used in a variety of food products, including processed meats, dairy products, baked goods, and snacks. They are primarily used to enhance the flavor, appearance, and texture of these products, making them more appealing to consumers.
However, some individuals may have a heightened sensitivity or allergy to Block 15 additives, leading to adverse reactions. The exact mechanism behind these reactions is not fully understood, but it is believed to involve an immune response triggered by the presence of these substances in the body.
It is important for individuals who have known sensitivities or allergies to LRA Additives/Preservatives, particularly Block 15, to carefully read food labels and avoid products that contain these substances. By being aware of their sensitivities and making informed choices, individuals can reduce the risk of experiencing adverse reactions and maintain their overall well-being.
Introduction to ELISA / ACT Biotechnologies
ELISA / ACT Biotechnologies is a renowned laboratory that specializes in the detection and analysis of various allergens and sensitivities. With a team of highly skilled scientists and cutting-edge technologies, they are at the forefront of research and innovation in the field of allergen detection.
At ELISA / ACT Biotechnologies, their mission is to provide accurate and reliable testing services to individuals and businesses alike. They understand the importance of identifying specific triggers that can cause adverse reactions, and their expertise lies in unraveling the complexities of allergens and sensitivities.
One of the key areas of focus for ELISA / ACT Biotechnologies is the detection of LRA Additives/Preservatives Block 15. This particular substance is commonly found in various food and beverage products and has been known to cause allergic reactions in some individuals. By utilizing their advanced laboratory techniques, ELISA / ACT Biotechnologies can accurately detect and quantify LRA Additives/Preservatives Block 15 in different samples.
The Science Behind ELISA
ELISA, or Enzyme-Linked Immunosorbent Assay, is a well-established laboratory technique used to detect and measure specific substances in biological samples. It is based on the principle of antigen-antibody interactions, where a target substance is captured and detected using a series of labeled antibodies.
In the case of LRA Additives/Preservatives Block 15, ELISA allows for accurate detection and quantification of this substance in various food and beverage samples. The process involves first immobilizing the target substance onto a solid surface, such as a microplate. Then, specific antibodies that are capable of binding to LRA Additives/Preservatives Block 15 are added to the sample.
If LRA Additives/Preservatives Block 15 is present in the sample, it will bind to the immobilized antibodies. The unbound substances are then washed away, and a secondary antibody, labeled with an enzyme, is added. This secondary antibody binds to the captured LRA Additives/Preservatives Block 15, forming a complex. By adding a substrate that reacts with the enzyme, a measurable signal is generated, indicating the presence and quantity of LRA Additives/Preservatives Block 15 in the sample.
ACT Biotechnologies: A Closer Look
ACT Biotechnologies, a partner of ELISA, specializes in providing comprehensive analysis and research on allergens and sensitivities. Their team of experts is dedicated to understanding the complexities of food-related triggers and developing innovative solutions to detect and manage them.
With a deep understanding of the molecular structure and behavior of allergenic substances, ACT Biotechnologies plays a crucial role in the identification of LRA Additives/Preservatives Block 15 in different food products and formulations. They employ advanced analytical techniques to analyze samples and determine the presence and concentration of this allergen.
ACT Biotechnologies' expertise extends beyond allergen detection. They also conduct research to better understand the mechanisms of allergic reactions and develop strategies for prevention and management. By collaborating with ELISA, they contribute to the advancement of knowledge and technology in the field of allergen detection and analysis.
In conclusion, ELISA / ACT Biotechnologies is a leading laboratory in the field of allergen detection and analysis. Their commitment to accuracy, reliability, and innovation makes them a trusted partner for individuals and businesses seeking comprehensive testing services. Through their expertise in ELISA and collaboration with ACT Biotechnologies, they continue to make significant contributions to the understanding and management of allergens and sensitivities.
Vega Test: An Overview
The Vega Test is an alternative method used for identifying sensitivities and intolerances. It utilizes a bioresonance device to measure the body's response to various substances and assess their potential impact on health.
The Vega Test, also known as electrodermal testing or electroacupuncture according to Voll (EAV), was developed by Dr. Reinhold Voll in the 1950s. It is based on the principles of traditional Chinese medicine and the concept of energy meridians in the body.
The test involves placing electrodes on specific acupuncture points on the skin, usually on the hands and feet. These acupuncture points are believed to correspond to different organs and systems in the body. The bioresonance device then sends low-level electrical currents through these points and measures the body's response.
The Principles of the Vega Test
The Vega Test works on the premise that substances, when introduced into the body, emit electromagnetic signals. By measuring the body's response to these signals, it is believed that potential sensitivities to LRA Additives/Preservatives Block 15 can be identified.
During the test, the bioresonance device is connected to a computer, which displays the readings and analyzes the data. The practitioner can then assess the body's response to different substances by observing changes in the electrical conductivity of the skin.
The Vega Test is often used to test for sensitivities to a wide range of substances, including foods, environmental allergens, toxins, and even emotional stressors. It is believed that when the body is exposed to a substance it is sensitive to, there will be a noticeable change in the electrical conductivity of the corresponding acupuncture point.
Based on the results of the Vega Test, the practitioner may recommend dietary changes, avoidance of certain substances, or specific treatments to address the identified sensitivities. It is important to note that the Vega Test is not intended to diagnose specific medical conditions, but rather to provide information about potential sensitivities and intolerances that may contribute to overall health issues.
Applications and Limitations of the Vega Test
While the Vega Test has gained popularity among some practitioners, it is important to note its limitations. The scientific validity and reproducibility of the Vega Test have been a subject of debate among experts. Critics argue that the test lacks a solid scientific foundation and that the observed changes in electrical conductivity may be influenced by various factors, such as skin moisture or the practitioner's technique.
Furthermore, the Vega Test has not been widely accepted by the mainstream medical community. Many medical professionals consider it to be a pseudoscientific practice and caution against relying solely on its results for making healthcare decisions.
Despite these limitations, proponents of the Vega Test argue that it can provide valuable insights into a person's overall health and help identify potential triggers for symptoms or chronic conditions. They believe that by addressing these sensitivities, individuals can improve their well-being and achieve better health outcomes.
It is important for individuals considering the Vega Test to approach it with an open mind and to consult with qualified healthcare professionals. A comprehensive evaluation, including a thorough medical history, physical examination, and appropriate diagnostic tests, should be conducted to ensure a holistic approach to healthcare.
Comparing ELISA / ACT Biotechnologies and Vega Test
When it comes to detecting LRA Additives/Preservatives Block 15, both ELISA / ACT Biotechnologies and the Vega Test offer their own unique approaches. Let's compare their methodologies and assess their accuracy and reliability.
Methodology Comparison
ELISA / ACT Biotechnologies employ a scientifically validated laboratory-based approach. The detection of LRA Additives/Preservatives Block 15 involves a series of precise steps and stringent quality control measures, ensuring reliable and reproducible results. On the other hand, the Vega Test relies on bioresonance technology, which lacks scientific consensus and may yield subjective outcomes.
Accuracy and Reliability: A Comparative Analysis
ELISA / ACT Biotechnologies' methodology has been extensively researched and validated. Their results are considered accurate and reliable, providing crucial data that can assist individuals in making informed decisions regarding their food choices. In contrast, due to the lack of scientific consensus surrounding the Vega Test, its accuracy and reliability remain uncertain.
Case Studies and Practical Applications
Case studies play an important role in understanding the practical application of these detection methods and their impact on real-world scenarios.
LRA Additives/Preservatives Block 15 Detection in Real-World Scenarios
Several studies have showcased the effectiveness of ELISA / ACT Biotechnologies in identifying LRA Additives/Preservatives Block 15 in a diverse range of food and beverage samples. Through accurate identification, individuals can avoid potential health risks associated with consuming these substances.
Vega Test in Clinical Practice
While the Vega Test has garnered interest among some healthcare practitioners, its use in clinical practice remains controversial. The lack of standardized protocols and scientific evidence makes it challenging to rely solely on the Vega Test for accurate detection of LRA Additives/Preservatives Block 15.
In conclusion, the detection of LRA Additives/Preservatives Block 15 plays a crucial role in the identification and management of potential health risks associated with these substances. ELISA / ACT Biotechnologies, with their scientifically validated approach, provides accurate and reliable results, aiding individuals in making informed decisions about their dietary choices. While the Vega Test may be of interest, its lack of scientific consensus and potential subjective outcomes warrant caution when using it as a sole detection method.