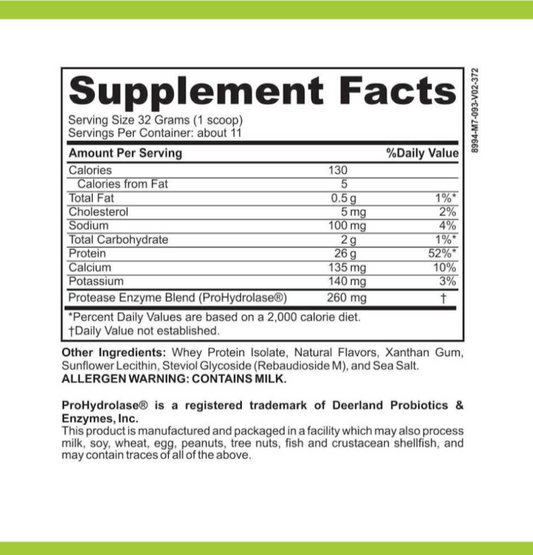
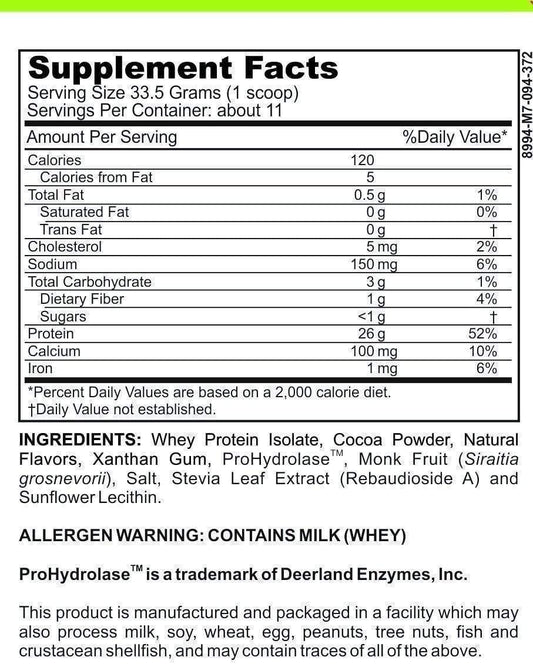
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test)
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test)
In the field of biotechnology, the detection and identification of various additives and preservatives are of utmost importance. One such method that has gained considerable attention is the LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies. This method is often compared to another widely used technique called the T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test). Let's take a closer look at the basics of LRA Additives/Preservatives Block 15, delve into the science behind ELISA / ACT Biotechnologies, and explore the features of the T.R.U.E. Test.
Understanding the Basics of LRA Additives/Preservatives Block 15
What are LRA Additives/Preservatives Block 15?
LRA Additives/Preservatives Block 15 is a revolutionary method used for the detection and quantification of additives and preservatives in biotechnological processes. These substances are commonly found in various industries, such as food and beverage, pharmaceuticals, and cosmetics. The Block 15 version of LRA (Lymphocyte Response Assay) specifically targets 15 common additives and preservatives, providing valuable insights into their presence and potential health effects.
When it comes to ensuring the safety and quality of products, the identification and analysis of additives and preservatives are of utmost importance. LRA Additives/Preservatives Block 15 is a cutting-edge technique that enables researchers and manufacturers to delve deeper into the composition of their products, allowing them to make informed decisions and take necessary actions to safeguard consumer health.
The detection and quantification of additives and preservatives are crucial in today's biotechnological landscape. With advancements in technology and the ever-growing demand for safe and sustainable products, LRA Additives/Preservatives Block 15 serves as a powerful tool in maintaining product integrity and meeting regulatory standards.
The Role of LRA Additives/Preservatives in Biotechnology
Additives and preservatives play a vital role in biotechnological applications. While they enhance product stability and shelf life, they may also pose risks to human health. LRA Additives/Preservatives Block 15 effectively screens for these substances, allowing researchers and manufacturers to make informed decisions regarding their usage.
In the food and beverage industry, additives and preservatives are commonly used to enhance flavors, improve texture, and extend product shelf life. However, the excessive use of these substances can have adverse effects on human health, ranging from allergic reactions to long-term health complications. With LRA Additives/Preservatives Block 15, biotechnologists can accurately assess the presence and concentration of these additives, enabling them to strike a balance between product quality and consumer safety.
Pharmaceutical and cosmetic industries also heavily rely on additives and preservatives to ensure the stability and efficacy of their products. However, the potential risks associated with these substances cannot be overlooked. LRA Additives/Preservatives Block 15 acts as a valuable screening tool, allowing researchers and manufacturers to identify any harmful additives or preservatives that may be present in their formulations. This knowledge empowers them to make necessary adjustments and develop safer alternatives without compromising product performance.
Moreover, LRA Additives/Preservatives Block 15 goes beyond mere detection. It provides insights into the potential health effects of these substances, allowing for a comprehensive assessment of their impact on human health. By understanding the risks associated with specific additives and preservatives, biotechnologists can make informed decisions about their usage, ensuring the safety and well-being of consumers.
Deep Dive into ELISA / ACT Biotechnologies
The Science Behind ELISA / ACT Biotechnologies
ELISA (Enzyme-Linked Immunosorbent Assay) is a widely used biochemical technique that detects and quantifies specific substances in various samples. It has revolutionized the field of diagnostics and research by providing a highly sensitive and specific method for detecting and measuring proteins, peptides, antibodies, and other biomolecules. ACT Biotechnologies, a leading biotech company, has taken this technology to the next level by applying it to the field of additives and preservatives detection.
ELISA / ACT Biotechnologies works by utilizing specific antibodies that bind to the target additive or preservative in the sample. These antibodies are designed to recognize and bind only to the specific substance of interest, ensuring accurate and reliable results. Once the antibodies have bound to the target substance, an enzyme is introduced, which produces a measurable signal. This signal indicates the presence and quantity of the additive or preservative in the sample, providing valuable information for quality control and regulatory compliance.
Applications and Uses of ELISA / ACT Biotechnologies
The applications of ELISA / ACT Biotechnologies are vast and diverse, making it a versatile tool for various industries. In the food and beverage industry, ELISA / ACT Biotechnologies can aid in quality control by detecting and quantifying additives and preservatives. This ensures that products meet regulatory standards and do not contain harmful or excessive amounts of these substances.
Furthermore, ELISA / ACT Biotechnologies can assist in identifying allergens in cosmetics, a crucial aspect of ensuring product safety for consumers. By detecting allergenic substances at trace levels, this technology helps cosmetic manufacturers avoid potential allergic reactions and maintain product integrity.
Pharmaceutical manufacturing also benefits from the accuracy and efficiency of ELISA / ACT Biotechnologies. It enables researchers and industry professionals to monitor and verify the presence of specific additives and preservatives in drug formulations, ensuring compliance with regulatory guidelines. This level of control and precision is vital for maintaining product efficacy and safety.
Moreover, ELISA / ACT Biotechnologies has found applications in environmental monitoring, veterinary diagnostics, and research laboratories. Its ability to detect and quantify target substances with high sensitivity and specificity makes it an invaluable tool for scientists and researchers in various fields.
In conclusion, ELISA / ACT Biotechnologies has revolutionized the field of additives and preservatives detection by harnessing the power of ELISA technology. Its applications span across industries, providing accurate and reliable results for quality control, regulatory compliance, and product safety. With its versatility and precision, ELISA / ACT Biotechnologies continues to drive advancements in diagnostics and research.
An Overview of T.R.U.E. Test (Thin-layer Rapid Use Epicutaneous Test)
The T.R.U.E. Test, also known as the Thin-layer Rapid Use Epicutaneous Test, is a specialized patch test designed to identify allergens in patients suspected of having allergic contact dermatitis. This innovative diagnostic tool has revolutionized the field of allergy detection by providing healthcare providers with a reliable method to pinpoint specific allergens causing patients' symptoms.
The procedure for the T.R.U.E. Test involves the application of small patches containing potential allergens onto the patient's skin. These patches are carefully placed on the patient's back, usually in a grid formation, to ensure comprehensive coverage. Once applied, the patches are left in place for a specified period, typically 48 hours, during which the patient is advised to avoid any activities that may compromise the integrity of the test.
During the observation period, healthcare providers closely monitor the patient's skin for any signs of a reaction. The patches act as a controlled exposure to potential allergens, allowing healthcare providers to assess the patient's sensitivity to each specific allergen. Any positive reactions, such as redness, swelling, or itching, are carefully documented and analyzed to determine the specific allergens responsible for the patient's symptoms.
The Principle and Procedure of T.R.U.E. Test
The T.R.U.E. Test operates on the principle of epicutaneous testing, which involves the application of allergens directly onto the skin to elicit a localized immune response. This type of testing is particularly effective in diagnosing allergic contact dermatitis, a form of delayed hypersensitivity reaction caused by exposure to allergens through direct skin contact. By identifying the specific allergens causing the patient's symptoms, healthcare providers can develop personalized treatment plans and preventive measures to alleviate the patient's condition.
When conducting the T.R.U.E. Test, healthcare providers adhere to a standardized procedure to ensure accurate and reliable results. First, the patient's medical history and symptoms are carefully evaluated to determine the appropriate panel of potential allergens to be tested. The selected allergens are then applied to separate patches, which are labeled and affixed to the patient's back using hypoallergenic adhesive tape.
After the patches have been secured, the patient is instructed to avoid excessive sweating, bathing, or any activities that may cause the patches to become dislodged. This precaution is necessary to maintain the integrity of the test and prevent false-negative results. Throughout the observation period, the patient is encouraged to report any discomfort or itching experienced at the patch sites.
At the end of the observation period, the patches are carefully removed, and the skin is thoroughly examined for any signs of a reaction. The healthcare provider evaluates the test results based on the presence and severity of reactions at each patch site. By comparing the patient's reactions to a standardized scoring system, healthcare providers can determine the specific allergens responsible for the patient's symptoms.
The Importance of T.R.U.E. Test in Allergy Detection
Allergies can significantly impact an individual's quality of life, causing discomfort, distress, and even life-threatening complications in severe cases. The T.R.U.E. Test plays a pivotal role in the accurate diagnosis of allergic contact dermatitis, enabling healthcare providers to identify the specific allergens triggering a patient's symptoms.
By pinpointing the exact allergens responsible for a patient's allergic contact dermatitis, healthcare providers can develop targeted treatment plans tailored to the patient's specific needs. This personalized approach minimizes the risk of unnecessary exposure to allergens and ensures the most effective management of the condition. Furthermore, the identification of specific allergens allows healthcare providers to provide patients with valuable information on allergen avoidance strategies, reducing the likelihood of future allergic reactions.
The T.R.U.E. Test also serves as a valuable tool in research and epidemiology, helping to identify trends and patterns in allergen sensitization. By analyzing the prevalence of specific allergens within a population, researchers can gain insights into the environmental factors contributing to allergic contact dermatitis and develop strategies for its prevention and control.
In conclusion, the T.R.U.E. Test is a critical diagnostic tool in the field of allergy detection. By accurately identifying the specific allergens causing allergic contact dermatitis, healthcare providers can offer targeted care, improve patients' quality of life, and contribute to ongoing research efforts in the field of allergies.
Comparing LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies and T.R.U.E. Test
Similarities and Differences: A Comparative Analysis
While both LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies and T.R.U.E. Test share a common goal of identifying substances, they differ in their approach and targeted applications. LRA Additives/Preservatives Block 15 focuses on detecting additives and preservatives in biotechnological processes, while the T.R.U.E. Test is primarily used for diagnosing allergic contact dermatitis in patients.
Strengths and Limitations of Each Method
LRA Additives/Preservatives Block 15 offers a comprehensive screening method specific to additives and preservatives common in various industries. Its ability to identify these substances aids in ensuring product safety. On the other hand, the T.R.U.E. Test excels in pinpointing specific allergens responsible for allergic reactions, enabling personalized treatment plans. However, it is limited to diagnosing allergic contact dermatitis and may not be applicable in all situations.
Real-World Applications and Case Studies
Case Study: LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies in Action
An example of the practical application of LRA Additives/Preservatives Block 15 is demonstrated in a food manufacturing company. By implementing this method, the company was able to identify and eliminate a potential allergen in one of their products, ensuring the safety and well-being of their customers.
Case Study: T.R.U.E. Test in Clinical Practice
In a dermatology clinic, the T.R.U.E. Test was instrumental in diagnosing a patient with allergic contact dermatitis. By identifying the specific allergens responsible for the patient's symptoms through the test, the healthcare provider was able to prescribe targeted treatment and prevent further exposure to allergens, improving the patient's condition and quality of life.
In conclusion, the LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies and the T.R.U.E. Test offer valuable insights into the identification and detection of substances in different contexts. While LRA Additives/Preservatives Block 15 focuses on additives and preservatives in biotechnological processes, the T.R.U.E. Test aids in diagnosing allergic contact dermatitis. Understanding the strengths, limitations, and real-world applications of these methods can assist researchers, healthcare providers, and industries in making informed decisions for the benefit of both product safety and patient care.