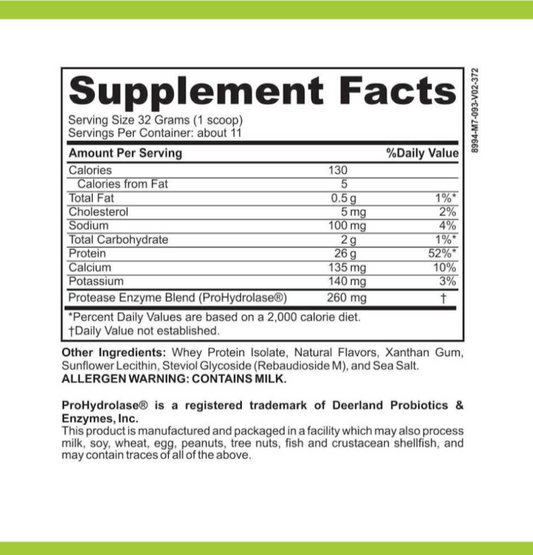
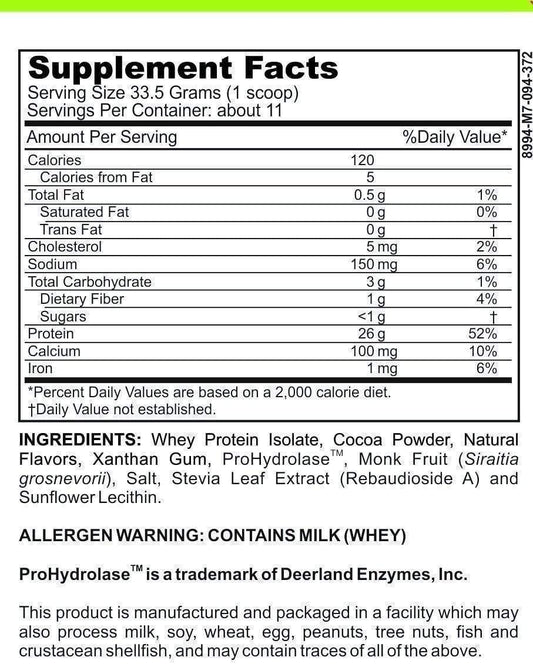
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs Polymerase Chain Reaction Testing
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs Polymerase Chain Reaction Testing
In the field of biotechnology, various testing methods are used to detect and analyze additives and preservatives. Among these methods, LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies and Polymerase Chain Reaction (PCR) Testing are two commonly employed techniques with unique advantages and applications. This article aims to provide a comprehensive comparison of these two methods, exploring their principles, methodologies, and future trends in biotechnological testing.
Understanding LRA Additives/Preservatives Block 15
What are LRA Additives/Preservatives Block 15? In the realm of biotechnology, LRA Additives/Preservatives Block 15 refers to a specific group of additives and preservatives that can be identified and quantified using ELISA / ACT Biotechnologies. This innovative testing method allows for the detection of a wide range of additives, ensuring the safety and quality of various products.
The Role of LRA Additives/Preservatives Block 15 in Biotechnology: LRA Additives/Preservatives Block 15 plays a crucial role in the biotechnological industry. With the increasing demand for clean and healthy products, the detection and analysis of additives and preservatives are essential. ELISA / ACT Biotechnologies utilizing LRA Additives/Preservatives Block 15 provides a reliable and efficient means to achieve this goal.
ELISA, which stands for Enzyme-Linked Immunosorbent Assay, is a widely used biochemical technique in the field of biotechnology. It is based on the principle of specific antibody-antigen interactions. By utilizing specific antibodies that bind to LRA Additives/Preservatives Block 15, ELISA can accurately detect and quantify the presence of these additives and preservatives in various samples.
The detection and analysis of additives and preservatives are of utmost importance in ensuring the safety and quality of food, pharmaceuticals, cosmetics, and other consumer products. LRA Additives/Preservatives Block 15, being a specific group of additives and preservatives, requires specialized testing methods to identify and quantify their presence. ELISA / ACT Biotechnologies provides a reliable and efficient means to achieve this, contributing to the overall safety and quality assurance of products in the biotechnological industry.
ACT Biotechnologies, a leading provider of innovative testing solutions, has developed ELISA kits specifically designed for the detection of LRA Additives/Preservatives Block 15. These kits offer high sensitivity and accuracy, enabling researchers and manufacturers to confidently assess the presence and concentration of LRA Additives/Preservatives Block 15 in their products.
By utilizing ELISA / ACT Biotechnologies for the detection of LRA Additives/Preservatives Block 15, companies can ensure regulatory compliance and meet the expectations of consumers who prioritize clean and healthy products. This advanced testing method provides a comprehensive analysis of additives and preservatives, allowing manufacturers to make informed decisions regarding the formulation and labeling of their products.
In conclusion, LRA Additives/Preservatives Block 15 are a specific group of additives and preservatives in the biotechnological industry. ELISA / ACT Biotechnologies offers a reliable and efficient means to detect and quantify the presence of these additives, ensuring the safety and quality of various products. With the increasing demand for clean and healthy products, the role of LRA Additives/Preservatives Block 15 in biotechnology is crucial, and the use of specialized testing methods contributes to overall safety and quality assurance.
An Overview of ELISA / ACT Biotechnologies
The Science Behind ELISA / ACT Biotechnologies: ELISA, which stands for Enzyme-Linked Immunosorbent Assay, is a widely used biochemical technique in the biotechnological field. It relies on the specific binding of antibodies to antigens, allowing for the detection and quantification of various substances, such as LRA Additives/Preservatives Block 15. ACT Biotechnologies has further developed and optimized ELISA techniques, enhancing their accuracy and sensitivity.
ELISA, as a powerful tool in the field of biotechnology, has revolutionized the way researchers and scientists analyze and detect substances. By utilizing the principles of immunology, ELISA enables the detection and measurement of specific substances in a variety of samples. This technique has become an indispensable part of research and diagnostic laboratories worldwide.
One of the key components of ELISA is the specific binding between antibodies and antigens. Antibodies are proteins produced by the immune system in response to the presence of foreign substances, known as antigens. In ELISA, antibodies are used to target and bind to specific antigens, allowing for their detection and quantification.
ACT Biotechnologies, a leading biotechnology company, has made significant advancements in ELISA techniques. Their research and development efforts have resulted in improved accuracy and sensitivity, making ELISA an even more reliable and precise method for detecting substances.
Applications of ELISA / ACT Biotechnologies in Testing: ELISA / ACT Biotechnologies have found numerous applications in the testing of additives and preservatives. By utilizing specific antibodies designed to target LRA Additives/Preservatives Block 15, ELISA / ACT Biotechnologies can precisely measure the presence and concentration of these substances in various samples.
The ability of ELISA / ACT Biotechnologies to detect and quantify LRA Additives/Preservatives Block 15 has significant implications in various industries. For instance, in the food industry, where the presence of additives and preservatives can have health and safety implications, ELISA / ACT Biotechnologies provide a reliable method for ensuring compliance with regulations and standards.
Moreover, ELISA / ACT Biotechnologies have also found applications in pharmaceutical research and development. By accurately measuring the concentration of LRA Additives/Preservatives Block 15 in drug formulations, researchers can assess the stability and efficacy of medications, ensuring their safety for human use.
Additionally, ELISA / ACT Biotechnologies have been instrumental in environmental monitoring. By detecting the presence of LRA Additives/Preservatives Block 15 in water or soil samples, scientists can assess the impact of human activities on the environment and take necessary measures to mitigate any potential risks.
In conclusion, ELISA / ACT Biotechnologies have revolutionized the field of biotechnology by providing a powerful tool for detecting and quantifying substances. With their enhanced accuracy and sensitivity, these techniques have found applications in various industries, including food, pharmaceutical, and environmental sectors. The ability to precisely measure the presence and concentration of LRA Additives/Preservatives Block 15 has significant implications for ensuring safety, compliance, and environmental protection.
Polymerase Chain Reaction Testing: A Detailed Look
The Process of Polymerase Chain Reaction Testing: Polymerase Chain Reaction, more commonly known as PCR, is a powerful molecular biology technique that amplifies specific regions of DNA. By using DNA primers and a heat-stable DNA polymerase enzyme, PCR can rapidly replicate and detect specific DNA sequences, including those associated with additives and preservatives.
Advantages of Polymerase Chain Reaction Testing: PCR offers several advantages in the field of biotechnological testing. It is highly sensitive, capable of detecting even minute quantities of target substances. Additionally, PCR is a versatile method, as it can detect a wide range of additives and preservatives. Furthermore, PCR testing can be conducted with relative ease and efficiency, making it a popular choice in many laboratories.
PCR has revolutionized the field of molecular biology and has become an indispensable tool for scientists and researchers alike. Its ability to amplify specific DNA sequences has opened up new avenues for studying genetic diseases, identifying genetic variations, and even solving crimes.
One of the key components of PCR is the DNA primer. Primers are short DNA sequences that are designed to bind to specific regions of the target DNA. These primers serve as the starting point for DNA replication during the PCR process. By designing primers that are complementary to the target DNA sequence, scientists can ensure that only the desired DNA region is amplified.
The heat-stable DNA polymerase enzyme used in PCR is another crucial element. This enzyme, often derived from thermophilic bacteria, is able to withstand the high temperatures required for DNA denaturation and replication. Without this heat-stable enzyme, PCR would not be possible, as the DNA polymerase used in traditional DNA replication would denature at the high temperatures used in the PCR process.
PCR testing is not limited to detecting additives and preservatives. It can also be used to detect genetic mutations, identify infectious diseases, and even determine paternity. The versatility of PCR makes it an invaluable tool in various fields, including medicine, agriculture, and forensic science.
Despite its numerous advantages, PCR does have some limitations. One limitation is the possibility of false positives or false negatives. Contamination during the PCR process or errors in primer design can lead to inaccurate results. Therefore, it is crucial for scientists to follow strict protocols and quality control measures to ensure the reliability and accuracy of PCR testing.
In conclusion, Polymerase Chain Reaction Testing is a powerful molecular biology technique that has revolutionized the field of biotechnological testing. Its ability to amplify specific DNA sequences with high sensitivity and efficiency has made it an indispensable tool in various scientific disciplines. With continued advancements in PCR technology, it is likely that this technique will continue to play a vital role in advancing our understanding of genetics and improving diagnostic capabilities.
Comparing ELISA / ACT Biotechnologies and Polymerase Chain Reaction Testing
Similarities and Differences in Methodologies: While ELISA / ACT Biotechnologies and PCR Testing share the common goal of detecting and analyzing additives and preservatives, they differ in their underlying methodologies. ELISA / ACT Biotechnologies relies on the binding of antibodies to antigens, while PCR amplifies specific DNA sequences. These distinct approaches allow for complementary benefits in terms of sensitivity and specificity.
Efficiency and Accuracy: A Comparative Analysis: When comparing the efficiency and accuracy of ELISA / ACT Biotechnologies and PCR Testing, it is essential to consider the specific requirements of the testing scenario. ELISA / ACT Biotechnologies excel in detecting LRA Additives/Preservatives Block 15, offering highly precise quantification. On the other hand, PCR Testing provides a broader spectrum of detection, making it suitable for identifying a wider range of additives and preservatives.
Practical Applications: Which Test to Use When? The choice between ELISA / ACT Biotechnologies and PCR Testing depends on the specific testing objectives and target substances. For the detection and analysis of LRA Additives/Preservatives Block 15, ELISA / ACT Biotechnologies are the ideal choice due to their high specificity and sensitivity. However, if a broader range of additives and preservatives need to be analyzed, PCR Testing would be more appropriate.
Future Trends in Biotechnological Testing
Innovations in ELISA / ACT Biotechnologies: The field of ELISA / ACT Biotechnologies is continuously evolving. Ongoing research and development efforts aim to further enhance the sensitivity and specificity of these techniques, allowing for more accurate and efficient detection of additives and preservatives. Additionally, the integration of automation and miniaturization technologies holds the promise of streamlined and high-throughput testing procedures.
Advances in Polymerase Chain Reaction Testing: PCR Testing is also the subject of ongoing advancements. Novel DNA amplification methods, such as digital PCR and Next-Generation Sequencing, offer increased sensitivity and multiplexing capabilities. These developments pave the way for more comprehensive and efficient detection of additives and preservatives.
The Impact of Emerging Technologies on Biotechnological Testing: Emerging technologies, such as microfluidics and nanotechnology, are poised to revolutionize biotechnological testing. These technologies enable the development of miniaturized and highly sensitive testing platforms, offering faster and more accurate detection of additives and preservatives. Additionally, the integration of artificial intelligence and machine learning algorithms promises to streamline data analysis and interpretation.
In conclusion, LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies and Polymerase Chain Reaction Testing are valuable methods for the detection and analysis of additives and preservatives. Each method presents unique advantages and applications. By understanding the principles, methodologies, and future trends of these techniques, researchers and industry professionals can make informed decisions when selecting the appropriate testing method for their specific needs.