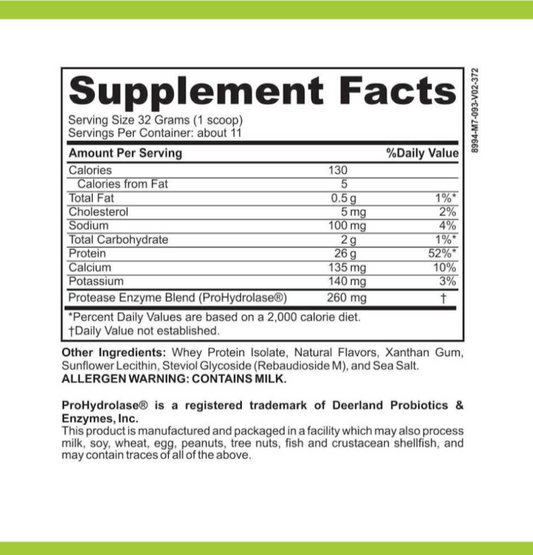
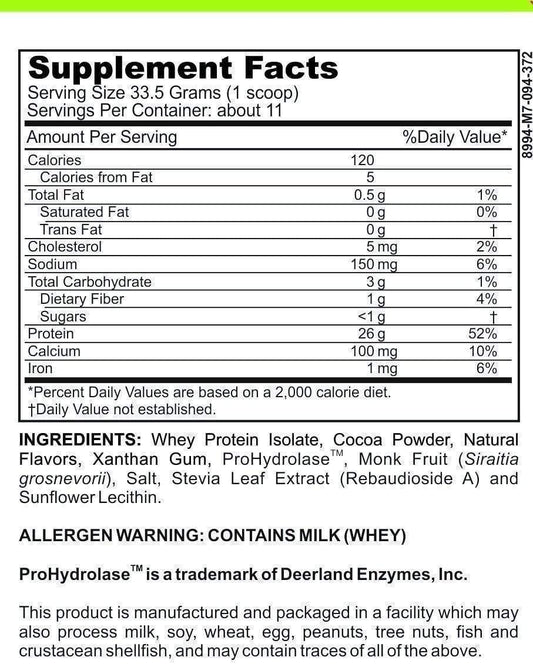
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs LEAP MRT
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs LEAP MRT
In the world of food safety and quality testing, the use of additives and preservatives is a crucial aspect. With increasing concerns about allergens and intolerances, it is essential to have effective methods to detect and analyze these substances. In this article, we will explore the role of LRA additives/preservatives block 15 and compare the testing capabilities of ELISA/ACT Biotechnologies and LEAP MRT.
Understanding LRA Additives/Preservatives Block 15
LRA additives are commonly used in the food industry to improve shelf life, maintain freshness, and enhance flavor. These additives, including preservatives, colorants, and antioxidants, are vital for ensuring the safety and quality of processed food products. LRA additives/preservatives block 15 is a specific combination of substances that have been carefully formulated to prevent spoilage and maintain product stability.
By effectively inhibiting the growth of microorganisms and slowing down enzymatic reactions, LRA additives/preservatives block 15 plays a crucial role in food preservation. However, it is essential to have accurate and reliable methods to identify and quantify these additives to ensure regulatory compliance and consumer safety.
The Role of LRA Additives in Food Preservation
LRA additives, including preservatives, possess antimicrobial properties that can extend the shelf life of food products. These substances inhibit the growth of pathogens, spoilage organisms, and molds, thereby reducing the risk of foodborne illnesses and maintaining product quality.
Furthermore, LRA additives can also control oxidative reactions, thereby preventing rancidity and maintaining the sensory attributes of food products. By keeping food fresh and safe for consumption, LRA additives make a significant contribution to the food industry.
For example, in the production of canned fruits, LRA additives such as citric acid are used to maintain the desired acidity level, preventing the growth of bacteria that can cause spoilage. This ensures that the canned fruits remain safe and delicious for an extended period.
In the case of bakery products, LRA additives like calcium propionate are commonly used to inhibit the growth of mold and extend the shelf life of bread and other baked goods. This allows consumers to enjoy these products for a longer time without compromising quality.
How ELISA / ACT Biotechnologies Contributes to LRA Additives
ELISA (Enzyme-Linked Immunosorbent Assay) is a widely used analytical technique in the food industry. It allows for the specific detection and quantification of various compounds, including LRA additives. ELISA tests developed by ACT Biotechnologies provide a reliable and efficient method for determining the presence and concentration of LRA additives in food samples.
With ELISA, food manufacturers and regulatory bodies can easily monitor the levels of LRA additives/preservatives block 15 in their products. This ensures compliance with regulatory standards and helps maintain consumer trust.
For instance, ACT Biotechnologies has developed ELISA kits that specifically target the LRA additives/preservatives block 15. These kits provide a simple and accurate way to measure the concentration of these additives in food samples, allowing manufacturers to ensure that their products meet the required standards.
By utilizing ELISA technology, food companies can take proactive measures to prevent the presence of excessive LRA additives/preservatives block 15, ensuring that consumers are not exposed to potentially harmful levels of these substances.
Furthermore, the use of ELISA in combination with other analytical techniques allows for comprehensive analysis of food samples, providing a more in-depth understanding of the presence and impact of LRA additives/preservatives block 15 in various food products.
Overall, the contribution of ELISA and ACT Biotechnologies in the detection and quantification of LRA additives/preservatives block 15 plays a crucial role in ensuring the safety and quality of food products, giving consumers peace of mind when making their food choices.
The Science Behind ELISA / ACT Biotechnologies
ELISA testing relies on the principle of highly specific antigen-antibody interactions. By utilizing specific antibodies that bind to the target LRA additives, ELISA can accurately detect their presence in food samples. This method offers high sensitivity and selectivity, making it an invaluable tool in food safety testing.
ELISA, which stands for Enzyme-Linked Immunosorbent Assay, is a widely used analytical technique in the field of biotechnology. It has revolutionized the way we detect and quantify LRA additives in food products. The science behind ELISA involves the precise interaction between antigens and antibodies, which are key components of the immune system.
Antigens are substances that can stimulate an immune response in the body. In the case of ELISA testing, these antigens are the LRA additives that we want to detect. Antibodies, on the other hand, are proteins produced by the immune system in response to the presence of antigens. They have a unique ability to bind specifically to their corresponding antigens.
The Process of ELISA Testing
ELISA testing involves several key steps. First, the target LRA additive is extracted from the food sample using appropriate solvents or reagents. This extraction process ensures that the additive is separated from other components of the sample, allowing for accurate detection.
Next, the extracted sample is added to a well plate or a solid-phase surface coated with the specific antibody. This antibody is carefully chosen to bind specifically to the LRA additive of interest. The well plate or solid-phase surface acts as a platform for the interaction between the antibody and the LRA additive.
If the LRA additive is present in the sample, it will bind to the immobilized antibody. This binding event is then detected by the addition of an enzyme-linked secondary antibody. This secondary antibody recognizes and binds to the primary antibody that is already bound to the LRA additive. The secondary antibody is conjugated with an enzyme that produces a color change or a fluorescent signal upon interaction with a substrate.
To measure the signal generated by the enzyme, a spectrophotometer or a fluorescence reader is used. These instruments can detect and quantify the intensity of the signal, providing valuable information about the concentration of the LRA additive in the sample.
Based on the intensity of the signal, the concentration of LRA additive can be determined. ELISA testing provides a quantitative measurement, allowing for accurate tracking of additive levels and compliance with regulatory limits. This information is crucial for food manufacturers, regulatory agencies, and testing laboratories to ensure the safety and quality of food products.
The Impact of ACT Biotechnologies on Food Safety
ACT Biotechnologies has made significant contributions to food safety through their ELISA testing solutions. Their expertise in developing highly specific antibodies and optimized ELISA protocols has revolutionized the detection and quantification of LRA additives.
ACT Biotechnologies has dedicated extensive research and development efforts to create reliable testing kits and equipment. These kits are designed to simplify the ELISA testing process, making it more accessible to a wide range of users. With their advanced technology, food manufacturers, regulatory agencies, and testing laboratories can efficiently analyze food samples for the presence of LRA additives.
By providing accurate and reliable results, ACT Biotechnologies has empowered the food industry to ensure the safety and quality of their products. The detection and quantification of LRA additives are crucial in maintaining consumer confidence and meeting regulatory requirements. With the help of ELISA testing, the industry can effectively mitigate risks associated with LRA additives, protect public health, and uphold the highest standards of food safety.
Comparing ELISA / ACT Biotechnologies and LEAP MRT
In addition to ELISA testing by ACT Biotechnologies, another method commonly employed in food testing is LEAP MRT (Molecular Resonance Technology). Both ELISA and LEAP MRT offer unique features and advantages, making them suitable for different applications.
The Functionality of LEAP MRT in Food Testing
LEAP MRT is based on the principle of nuclear magnetic resonance (NMR), allowing for the identification and quantification of various compounds. Unlike ELISA, which relies on antigen-antibody interactions, LEAP MRT directly measures the signals emitted by the target molecules.
This non-invasive technology provides rapid and comprehensive analysis, making it useful for large-scale screening and broad-spectrum detection. LEAP MRT offers a holistic view of the sample composition, enabling the identification of multiple additives simultaneously.
Strengths and Weaknesses of ELISA / ACT Biotechnologies and LEAP MRT
ELISA testing by ACT Biotechnologies excels in its ability to quantitatively measure the concentration of specific LRA additives/preservatives block 15. It offers high sensitivity, selectivity, and ease of use. However, ELISA requires specific antibodies and optimized protocols for each target compound, limiting its flexibility.
On the other hand, LEAP MRT provides a broader analysis of the entire chemical composition of the sample, allowing for comprehensive characterization. It can detect various compounds simultaneously, making it ideal for exploratory research and large-scale screening. However, it may not offer the same level of specificity and quantitative accuracy as ELISA in terms of LRA additive detection.
Case Studies of LRA Additives/Preservatives Block 15 Usage
To understand the real-world applications of ELISA/ACT Biotechnologies and LEAP MRT in testing LRA additives, let's explore some case studies.
Real-world Applications of ELISA / ACT Biotechnologies
In a study conducted by a leading food manufacturer, ELISA testing by ACT Biotechnologies was used to monitor the levels of LRA additives/preservatives block 15 in their processed meat products. The results obtained with ELISA testing provided crucial information for optimizing the production process and ensuring compliance with regulatory limits.
Another case study involved a regulatory agency responsible for monitoring imported food products. By utilizing ELISA testing, they were able to quickly identify and quantify LRA additives in a range of imported food samples, ensuring their safety and preventing potential health risks for consumers.
Practical Examples of LEAP MRT in Action
In a research institution focused on food product development, LEAP MRT was used for a comprehensive analysis of different food samples. By detecting a wide range of additives and ingredients, LEAP MRT helped researchers gain insights into the overall quality and composition of the tested products.
A food testing laboratory used LEAP MRT for rapid screening of food samples, allowing them to detect unwanted additives and potential contaminants efficiently. This enabled prompt actions to minimize risks and maintain food safety standards.
Future Trends in Food Additives and Preservatives Testing
The field of food safety testing is continuously evolving, driven by advances in technology and regulatory requirements. The future holds exciting possibilities for ELISA/ACT Biotechnologies and LEAP MRT in detecting LRA additives/preservatives block 15.
Innovations in ELISA / ACT Biotechnologies
ACT Biotechnologies is continuously working to enhance their ELISA testing solutions. This includes the development of multiplex ELISA assays capable of detecting multiple LRA additives simultaneously, reducing time and resources required for testing.
Furthermore, there is ongoing research to optimize ELISA protocols for improved performance and to broaden the range of detectable LRA additives. These innovations will further enhance the capability of ELISA/ACT Biotechnologies in ensuring food safety and quality.
The Future of LEAP MRT in Food Safety
As technological advancements continue, LEAP MRT is expected to play a vital role in food safety testing. Improvements in sensitivity and data analysis algorithms will enable more accurate and reliable identification of LRA additives. Additionally, integration with other analytical techniques may further expand the capabilities of LEAP MRT.
The future of LEAP MRT lies in its ability to provide rapid and comprehensive analysis, ensuring the safety and quality of food products in an increasingly complex and globalized food supply chain.
In conclusion, the detection and analysis of LRA additives/preservatives block 15 in food products is a critical aspect of food safety. ELISA/ACT Biotechnologies and LEAP MRT offer distinct approaches, with ELISA providing quantitative and specific measurements while LEAP MRT offers broader characterization. Both methods have their strengths and applications, contributing to the overall goal of ensuring safe and high-quality food for consumers. The future holds exciting developments in these technologies, further advancing the field of food additives and preservatives testing.