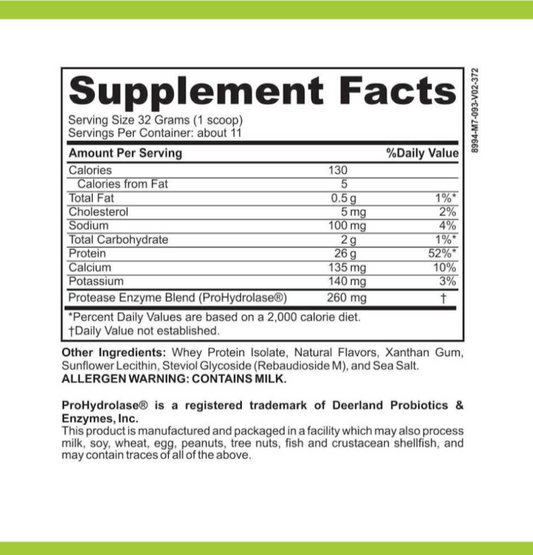
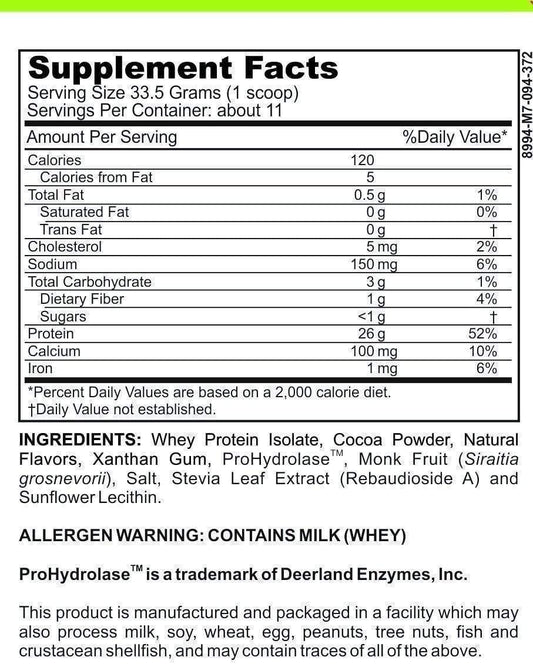
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs ACT Testing
LRA Additives/Preservatives Block 15 by ELISA / ACT Biotechnologies Vs ACT Testing
LRA additives/preservatives block 15 refers to a specific type of testing used in the food and pharmaceutical industries to detect the presence of additives and preservatives that may cause adverse reactions in individuals. These additives and preservatives can include chemicals such as artificial flavors, coloring agents, and stabilizers, among others. The purpose of this article is to provide an understanding of the basics of LRA additives/preservatives block 15, explore the science behind ELISA/ACT Biotechnologies, compare it to ACT testing, examine real-world applications, and discuss the future of this type of testing.
Understanding the Basics of LRA Additives/Preservatives Block 15
Before delving into the details of LRA additives/preservatives block 15 testing, it is important to grasp the concept of what additives and preservatives are. These substances are commonly added to processed foods and pharmaceutical products to enhance flavor, appearance, or shelf life. However, they can also lead to various negative health effects in some individuals.
When it comes to additives, there are a wide range of options available. These can include artificial sweeteners, colorings, flavor enhancers, and stabilizers. Preservatives, on the other hand, are substances that prevent the growth of microorganisms and help to extend the shelf life of products. Some common preservatives include sulfites, benzoates, and nitrates.
LRA additives/preservatives block 15 is specifically designed to identify and quantify the presence of these additives and preservatives in a given sample. This testing method aims to provide insight into potential allergic or adverse reactions that individuals may experience as a result of consuming or using products containing these substances.
What are LRA Additives/Preservatives Block 15?
LRA additives/preservatives block 15 is a type of testing that utilizes ELISA/ACT Biotechnologies to identify and measure the presence and levels of specific additives and preservatives in a sample. The technology relies on advanced immunoassay techniques to detect and analyze the antibodies produced by the body when exposed to these substances.
By measuring the antibody response to individual additives and preservatives, LRA additives/preservatives block 15 testing can provide crucial information about potential adverse reactions and sensitivities in individuals. This helps in identifying which specific additives or preservatives may be causing negative health effects in a person.
The testing process involves collecting a sample from the individual, such as blood or urine, and then analyzing it in a laboratory setting. The sample is exposed to a panel of additives and preservatives, and any antibody reactions are measured and recorded. This comprehensive analysis allows for a thorough understanding of an individual's sensitivities and helps guide dietary or pharmaceutical choices.
The Role of LRA Additives/Preservatives in Food and Pharmaceuticals
Additives and preservatives play a vital role in the food and pharmaceutical industries. They are used to enhance the taste, texture, and appearance of products, as well as to extend their shelf life. However, for certain individuals, these additives and preservatives can trigger allergic reactions, intolerances, or sensitivities.
For example, some people may experience symptoms such as headaches, digestive issues, or skin rashes when exposed to certain additives or preservatives. These reactions can vary in severity, with some individuals experiencing mild discomfort and others facing more serious health complications.
LRA additives/preservatives block 15 testing aims to address these concerns by providing a comprehensive analysis of the specific substances that may be causing adverse reactions. This information allows individuals, healthcare professionals, and manufacturers to make informed decisions about the ingredients used in food and pharmaceutical products, ensuring the safety and well-being of consumers.
By understanding an individual's sensitivities and reactions to specific additives and preservatives, it becomes possible to develop tailored dietary or pharmaceutical plans that minimize the risk of adverse effects. This personalized approach to consumption can greatly improve the quality of life for those who are sensitive to certain substances.
Furthermore, the data collected through LRA additives/preservatives block 15 testing can also contribute to ongoing research and development efforts. By identifying trends and patterns in sensitivities, scientists and manufacturers can work towards creating safer and more inclusive products for all consumers.
The Science Behind ELISA / ACT Biotechnologies
ELISA/ACT Biotechnologies is a cutting-edge technology that forms the foundation of LRA additives/preservatives block 15 testing. This innovative approach combines the principles of enzyme-linked immunosorbent assay (ELISA) and antigen-specific lymphocyte stimulation testing (ACT) to detect and analyze immune responses to additives and preservatives.
This groundbreaking technology has revolutionized the way we understand and diagnose sensitivities and allergies to additives and preservatives. By utilizing the power of ELISA and ACT, scientists and healthcare professionals are able to gain valuable insights into the immune system's response to these substances, ultimately leading to improved patient care and better management of dietary and medication choices.
An Overview of ELISA / ACT Biotechnologies
ELISA/ACT Biotechnologies involves a two-step process. In the first step, ELISA, antibodies specific to the target additives or preservatives are introduced to the sample. These antibodies are designed to bind specifically to the target substances, allowing for their detection. If the additives or preservatives are present in the sample, they bind to the antibodies, forming an antigen-antibody complex.
This binding interaction is then detected using colorimetric or fluorescent methods. These methods allow scientists to visualize and quantify the presence of the target substances in the sample, providing valuable information about their concentration.
The second step, ACT, involves the stimulation of lymphocytes in the blood sample with the antigens present in the additives or preservatives. Lymphocytes are a type of white blood cell that plays a crucial role in the immune response. By exposing the lymphocytes to the antigens, scientists can measure the immune response and identify any sensitivities or allergies.
This step is particularly important as it allows for a comprehensive analysis of the individual's immune system response to the specific additives or preservatives. By measuring the activation and proliferation of lymphocytes, scientists can determine the level of immune reactivity to these substances, providing valuable information about potential sensitivities or allergies.
How ELISA / ACT Biotechnologies Works with LRA Additives/Preservatives Block 15
LRA additives/preservatives block 15 testing utilizes the ELISA/ACT Biotechnologies platform to provide a comprehensive analysis of individual additives and preservatives. This testing method offers a wide range of benefits, including enhanced accuracy and specificity.
The process begins by exposing the sample to specific antigens, which are designed to mimic the additives or preservatives of interest. These antigens are carefully selected to ensure that they accurately represent the target substances. The sample is then incubated, allowing for the interaction between the antigens and any antibodies present in the sample.
Following the incubation period, the sample is subjected to ELISA methodology, where the presence and concentration of the target substances are measured. This step provides quantitative data about the individual additives or preservatives, allowing for a detailed analysis of their presence in the sample.
ACT testing is then performed by stimulating lymphocytes in the sample with the antigens of the identified additives or preservatives. This step allows for the measurement of the immune response and the identification of any sensitivities or allergies. By analyzing the activation and proliferation of lymphocytes, scientists can determine the level of immune reactivity to the specific substances, providing valuable insights into an individual's immune system response.
The information obtained from ELISA/ACT Biotechnologies testing with LRA additives/preservatives block 15 is crucial for individuals and healthcare professionals. It helps them make informed decisions regarding the inclusion or avoidance of certain additives or preservatives in their diet or medication. By understanding an individual's immune response to these substances, personalized dietary and medication plans can be developed to promote overall health and well-being.
Comparing ELISA / ACT Biotechnologies and ACT Testing
While ELISA/ACT Biotechnologies and ACT testing serve a similar purpose, they differ in their approaches and methodologies for identifying and measuring immune responses to additives and preservatives.
The Key Differences Between ELISA / ACT Biotechnologies and ACT Testing
ELISA/ACT Biotechnologies combines the sensitivity of ELISA with the specificity of ACT, providing a more comprehensive analysis of specific additives and preservatives. It measures both the presence and quantity of antibodies, allowing for a more accurate assessment of sensitivities or allergies.
In contrast, traditional ACT testing focuses solely on the stimulation and measurement of lymphocyte responses. While effective, it may not provide the same level of detailed information regarding individual additives and preservatives that ELISA/ACT Biotechnologies offers.
The Pros and Cons of ELISA / ACT Biotechnologies and ACT Testing
ELISA/ACT Biotechnologies has several advantages over ACT testing. Its ability to detect and quantify antibodies associated with specific additives and preservatives provides a more precise and comprehensive assessment. This allows for personalized dietary or medication recommendations, minimizing the risk of adverse reactions.
However, ELISA/ACT Biotechnologies may require specialized equipment and expertise, making it less accessible than traditional ACT testing. Additionally, the cost may be higher, which could limit its widespread adoption.
Case Studies and Real-World Applications
To understand the practical applications of LRA additives/preservatives block 15 testing, it is essential to examine real-world scenarios where this technology has proven beneficial.
How ELISA / ACT Biotechnologies is Used in Real-World Scenarios
One notable application of ELISA/ACT Biotechnologies is in identifying and managing food allergies and sensitivities. By analyzing individual additives and preservatives, this testing method helps individuals and healthcare professionals pinpoint the specific substances that trigger adverse reactions, facilitating the development of customized dietary plans.
Moreover, ELISA/ACT Biotechnologies is instrumental in assessing the safety and efficacy of pharmaceutical products. It enables the identification of potential additives or preservatives that may induce allergic reactions or adverse drug interactions, ensuring the well-being of patients.
Examples of ACT Testing in Action
ACT testing has also found application in identifying sensitivities and intolerances to various substances. For instance, it has been used to assess reactions to environmental allergens like pollen or pet dander, as well as to chemicals in the workplace, aiding in the prevention and management of allergies.
ACT testing has also proved useful in diagnosing autoimmune conditions and determining appropriate treatment options. By measuring lymphocyte responses to specific antigens present in these conditions, it helps inform medical decisions and personalize patient care.
The Future of LRA Additives/Preservatives Block 15 Testing
The field of LRA additives/preservatives block 15 testing is constantly evolving, with new technologies and advancements on the horizon.
Emerging Trends in LRA Additives/Preservatives Block 15 Testing
One emerging trend is the development of rapid and portable testing devices for LRA additives/preservatives block 15 analysis. These advancements aim to provide quick and accessible results, enabling on-the-spot evaluations of products and immediate decision-making.
Additionally, researchers continue to investigate new additives and preservatives to include in testing panels. By expanding the range of substances analyzed, LRA additives/preservatives block 15 testing can deliver more comprehensive and accurate results, enhancing its utility in identifying potential allergens and sensitivities.
The Potential Impact of New Technologies on LRA Additives/Preservatives Block 15 Testing
New technologies, such as machine learning and artificial intelligence, have the potential to revolutionize LRA additives/preservatives block 15 testing. By analyzing vast amounts of data and patterns, these technologies can enhance the accuracy and efficiency of testing methods, making personalized assessments more accessible and precise.
Furthermore, advancements in molecular biology techniques may allow for more targeted and specific testing, further improving the identification and analysis of individual additives and preservatives.
In summary, LRA additives/preservatives block 15 testing, facilitated by ELISA/ACT Biotechnologies, provides valuable insights into potential adverse reactions to specific additives and preservatives. Its meticulous analysis of individual substances allows for personalized recommendations, minimizing the risk of adverse effects. While ELISA/ACT Biotechnologies and ACT testing differ in their approaches, both contribute to the identification and management of sensitivities and allergies. Real-world applications showcase the practical benefits of this testing, ranging from food allergy management to pharmaceutical safety. The future of LRA additives/preservatives block 15 testing holds promising advancements, such as portable devices and technological innovations, further improving accuracy and accessibility.