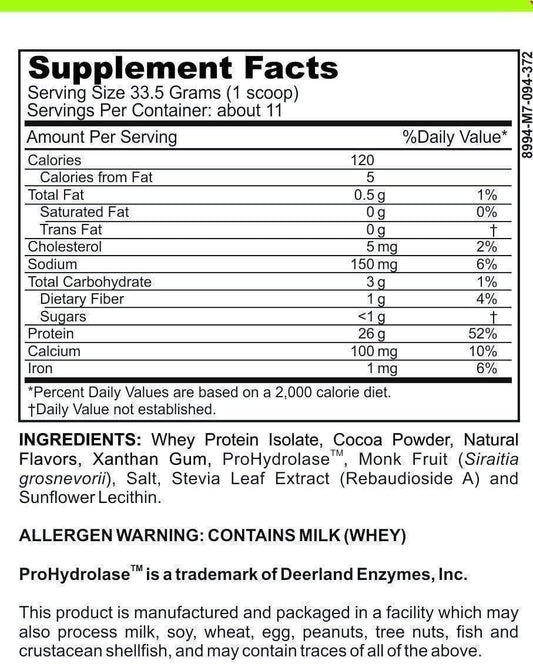
LRA Comprehensive 317 Panel by ELISA / ACT Biotechnologies Vs LEAPs Test
In the field of allergy testing, two popular methods have emerged as reliable tools for identifying and managing adverse immune reactions: the LRA Comprehensive 317 Panel by ELISA/ACT Biotechnologies and the LEAPs Test. These tests offer valuable insights into the body's immune response and help healthcare providers develop effective treatment strategies. In this article, we will delve into the intricacies of these tests, their advantages, limitations, and compare them to understand which one might be the better choice for patients.
Understanding the LRA Comprehensive 317 Panel by ELISA
Before delving into the comparison, let's begin by examining the LRA Comprehensive 317 Panel by ELISA and understanding its purpose and functioning.
What is the LRA Comprehensive 317 Panel?
The LRA Comprehensive 317 Panel is a sophisticated and comprehensive allergy test designed to identify delayed hypersensitivity (type IV) reactions caused by various environmental and dietary allergens. It screens for a wide range of triggers, including foods, chemicals, molds, inhalants, and more.
How Does the LRA Comprehensive 317 Panel Work?
This test works by measuring the patient's immune response against a panel of 317 different allergens. Blood samples collected from patients are exposed to these allergens in a controlled laboratory setting. The immune system's reactivity is assessed by measuring the production of pro-inflammatory substances, such as cytokines and antibodies, which indicate an immune reaction.
Applications and Benefits of the LRA Comprehensive 317 Panel
The LRA Comprehensive 317 Panel offers several advantages. Firstly, it provides a comprehensive analysis of an individual's immune reactivity to a broad range of potential allergens. This detailed information helps healthcare providers develop personalized treatment plans, including dietary modifications, environmental changes, and targeted therapies.
Furthermore, this test enables the identification of hidden or delayed food allergies that may not manifest obvious symptoms but still contribute to chronic health conditions. By addressing these underlying allergens, patients may experience improvements in various areas, such as digestion, energy levels, and overall well-being.
In addition, the LRA Comprehensive 317 Panel can aid in managing chronic inflammatory conditions like arthritis, irritable bowel syndrome (IBS), and autoimmune disorders. By identifying and addressing the triggers, patients may experience reduced inflammation and improved symptom control.
Moreover, the LRA Comprehensive 317 Panel is a valuable tool for individuals who have been struggling with unexplained symptoms or have not found relief from conventional allergy testing methods. The extensive range of allergens tested in this panel allows for a more comprehensive assessment, increasing the likelihood of identifying the specific triggers responsible for an individual's symptoms.
It is important to note that the LRA Comprehensive 317 Panel is conducted using the enzyme-linked immunosorbent assay (ELISA) technique, a widely accepted and reliable method in the field of immunology. ELISA provides accurate and quantitative results, ensuring the reliability of the test outcomes.
Overall, the LRA Comprehensive 317 Panel by ELISA is a cutting-edge allergy test that offers a comprehensive analysis of an individual's immune reactivity to a wide range of potential allergens. Its applications extend beyond the identification of allergies, as it can also aid in managing chronic inflammatory conditions. By providing detailed information and personalized treatment plans, this test empowers healthcare providers and patients to take proactive steps towards improved health and well-being.
An In-depth Look at ACT Biotechnologies
Welcome to this comprehensive exploration of ACT Biotechnologies, the pioneering company responsible for the development of the LRA Comprehensive 317 Panel. By delving into the role of ACT Biotechnologies, we can gain a deeper understanding of the test's reliability and credibility, as well as the advantages it offers in the field of allergy testing.
The Role of ACT Biotechnologies in Allergy Testing
ACT Biotechnologies is a renowned leader in the realm of allergy and immunology. With their vast expertise, they have been instrumental in creating advanced diagnostic tools that empower healthcare providers to accurately identify and address allergic reactions. Their unwavering commitment to research and innovation has been the driving force behind their continuous efforts to enhance the reliability and effectiveness of allergy testing, exemplified by the groundbreaking LRA Comprehensive 317 Panel.
ACT Biotechnologies' comprehensive approach to allergy testing encompasses a wide range of factors. They meticulously analyze the intricate mechanisms of the immune system, employing cutting-edge technologies to unravel the complexities of allergic reactions. By deciphering the underlying causes and triggers of allergies, ACT Biotechnologies enables healthcare professionals to develop tailored treatment plans that address the root causes, rather than merely alleviating symptoms.
Advantages of Using ACT Biotechnologies
One of the key advantages of utilizing ACT Biotechnologies' testing methods is the unparalleled accuracy and reliability of their results. The company's unwavering dedication to scientific rigor is evident in the extensive research and stringent quality control measures they employ. This ensures that the outcomes of their tests are precise, reproducible, and provide patients and healthcare providers with a solid foundation for developing effective treatment strategies.
In addition to their commitment to accuracy, ACT Biotechnologies places great emphasis on the importance of ongoing research and updates to continually improve their testing methods. By staying at the forefront of scientific advancements in the field of allergy testing, they ensure that their tests remain highly accurate and reliable, even as new discoveries emerge. This dedication to innovation ensures that patients and healthcare providers can rely on ACT Biotechnologies' tests to incorporate the latest scientific knowledge, resulting in enhanced accuracy and efficacy.
Furthermore, ACT Biotechnologies understands that every patient is unique, and their approach to allergy testing reflects this understanding. They recognize that different individuals may have varying sensitivities and responses to allergens, and their tests are designed to account for these individual differences. By tailoring their testing methods to the specific needs of each patient, ACT Biotechnologies provides personalized and comprehensive insights that facilitate targeted treatment plans.
In conclusion, ACT Biotechnologies stands at the forefront of allergy testing, driven by a passion for scientific excellence and a commitment to improving the lives of allergy sufferers. Through their relentless pursuit of innovation and accuracy, they continue to revolutionize the field, empowering healthcare providers with the tools they need to provide optimal care for patients with allergies.
Introduction to LEAPs Test
Now that we have explored the LRA Comprehensive 317 Panel, let's turn our attention to the LEAPs Test, another widely used method for investigating delayed immune reactions.
The Science Behind the LEAPs Test
The LEAPs Test, or "Lymphocyte Exploratory Antigen Presentation Test," is based on the principle of antigen presentation. In simple terms, this test measures the proliferation of lymphocytes (a type of white blood cell) in response to specific allergens. By analyzing the immune system's reaction using lymphocytes, the LEAPs Test helps identify hidden or delayed allergens that may contribute to chronic health conditions.
Pros and Cons of the LEAPs Test
Like any diagnostic test, the LEAPs Test has its own set of advantages and limitations to consider.
On the positive side, the LEAPs Test offers a non-invasive and relatively painless method for assessing delayed immune reactions. It can identify allergens that may not be detectable through traditional skin prick tests or IgE blood tests, making it a valuable tool for individuals with chronic health conditions whose causative factors may be elusive.
However, it is essential to acknowledge that the LEAPs Test is a relatively new technique, and its scientific validity is still being established. While preliminary research looks promising, further studies are needed to validate its efficacy and accuracy compared to other established allergy testing methods.
LRA Comprehensive 317 Panel by ELISA / ACT Biotechnologies Vs LEAPs Test: A Comparative Analysis
Now that we have gained an understanding of both the LRA Comprehensive 317 Panel and the LEAPs Test let's compare them across several parameters to evaluate their respective merits.
Accuracy Comparison
Accuracy is a crucial aspect when it comes to allergy testing. In terms of accuracy, the LRA Comprehensive 317 Panel by ELISA/ACT Biotechnologies holds a strong track record. Extensive research and validation studies have consistently demonstrated its reliability and precision.
While the LEAPs Test shows promising potential, further research and validation are necessary to establish its accuracy compared to the LRA Comprehensive 317 Panel.
Cost-effectiveness Analysis
When considering diagnostic tests, cost-effectiveness is an important factor to consider for both patients and healthcare providers. The LRA Comprehensive 317 Panel is an investment due to its comprehensive nature and diverse panel of allergens. However, the upfront cost may be outweighed by its ability to provide targeted treatment strategies, potentially reducing the overall healthcare expenses associated with managing chronic health conditions.
On the other hand, the LEAPs Test may be a more cost-effective option for individuals specifically seeking to identify hidden or delayed allergens without the desire for a comprehensive analysis.
Patient Comfort and Convenience
Patient comfort and convenience play pivotal roles in the acceptance and adoption of allergy testing methods. The LRA Comprehensive 317 Panel requires a blood sample, which may cause slight discomfort but is generally well-tolerated by patients. Moreover, the in-depth analysis and comprehensive report provided by the LRA Comprehensive 317 Panel justify the minor inconvenience.
The LEAPs Test, with its non-invasive approach that involves sending a cheek swab for analysis, offers a convenient and hassle-free experience for patients.
Expert Opinions and Case Studies
It is valuable to consider the opinions of medical experts who have worked with both the LRA Comprehensive 317 Panel and the LEAPs Test to gain insights into their real-world efficacy and applications.
Medical Experts' Views on LRA Comprehensive 317 Panel and LEAPs Test
Medical experts widely recognize and appreciate the comprehensive nature of the LRA Comprehensive 317 Panel. Its ability to identify hidden or delayed allergens, combined with personalized treatment strategies, allows for targeted interventions with potential long-term benefits for patients.
Similarly, medical experts acknowledge the potential of the LEAPs Test in uncovering delayed immune reactions. However, due to its relative novelty, many experts advocate for further research and validation to solidify its position as a reliable diagnostic tool.
Case Studies Illustrating the Effectiveness of Both Tests
Several case studies have documented the effectiveness of both the LRA Comprehensive 317 Panel and the LEAPs Test in improving patients' quality of life.
Case studies involving patients who underwent the LRA Comprehensive 317 Panel demonstrated significant improvements in various areas, such as digestion, energy levels, joint pain reduction, and overall symptom control. These positive outcomes highlight the test's potential to address chronic health conditions by identifying and treating underlying allergens.
Similarly, case studies focusing on the LEAPs Test have showcased improvements in patients' well-being by identifying and eliminating hidden allergens that contributed to chronic health issues.
In conclusion, the choice between the LRA Comprehensive 317 Panel by ELISA/ACT Biotechnologies and the LEAPs Test ultimately depends on individual patient needs, the complexity of their conditions, and the level of comprehensive analysis desired. Both tests offer valuable insights into delayed immune reactions and assist healthcare providers in developing effective treatment strategies. Patients are encouraged to consult with their healthcare providers to determine the most suitable testing option for their specific situation.