Is Calcium Peroxide Dairy Free
Is Calcium Peroxide Dairy Free
Calcium peroxide is a commonly used material in various industries, including food production. Its chemical composition and properties make it a versatile ingredient, but is calcium peroxide dairy free? In order to answer this question, it is important to understand what calcium peroxide is and how it is used.
Understanding Calcium Peroxide
Calcium peroxide is a white, odorless powder that is most commonly known for its ability to release oxygen when it comes into contact with water. Its chemical formula, CaO2, indicates the presence of calcium and oxygen in its structure. This compound is often used as a source of oxygen in different applications, thanks to its stability and controlled release characteristics.
When calcium peroxide is exposed to moisture, it slowly decomposes, releasing molecular oxygen. This oxygen-release property is due to the bond between two oxygen atoms and a calcium atom in its chemical composition. The gradual release of oxygen makes calcium peroxide useful in various industries, including the food industry.
The Chemical Composition of Calcium Peroxide
The chemical composition of calcium peroxide consists of two oxygen atoms bonded to a calcium atom. This bond allows the compound to slowly decompose when exposed to moisture, releasing molecular oxygen. It is this oxygen-release property that makes calcium peroxide useful in various industries, including the food industry.
Calcium peroxide is a stable compound, ensuring controlled release of oxygen. This stability is crucial in applications where a steady supply of oxygen is required. The presence of calcium in its composition also contributes to its stability, preventing rapid decomposition.
Furthermore, the white, odorless powder form of calcium peroxide makes it easy to handle and incorporate into different processes. Its fine particle size allows for uniform distribution, ensuring consistent oxygen release throughout the desired application.
Common Uses of Calcium Peroxide
Calcium peroxide has a wide range of applications, both in food and non-food industries. In the food industry, it is primarily used as a food additive. Its oxygen-releasing capabilities make it suitable for enhancing dough quality, as well as for improving the texture and appearance of baked goods.
When added to dough, calcium peroxide reacts with water, releasing oxygen. This oxygen reacts with gluten proteins, strengthening the dough structure and improving its elasticity. As a result, baked goods have a better texture, with increased volume and improved crumb structure.
In addition to enhancing dough quality, calcium peroxide can also be used in food storage and preservation. Its antimicrobial properties help inhibit the growth of spoilage-causing microorganisms, extending the shelf life of food products. This makes it a valuable tool in maintaining food quality and reducing waste.
Beyond the food industry, calcium peroxide finds applications in various non-food industries as well. It is commonly used in environmental remediation to treat contaminated soil and water. The oxygen released by calcium peroxide helps break down organic pollutants, facilitating the cleanup process.
Furthermore, calcium peroxide is utilized in the textile industry for bleaching purposes. Its controlled release of oxygen allows for effective and controlled bleaching of fabrics, ensuring desired color results without damaging the textile fibers.
Overall, calcium peroxide's ability to release oxygen in a controlled manner makes it a versatile compound with numerous applications. Whether it is improving dough quality, preserving food, or aiding in environmental cleanup, calcium peroxide plays a vital role in various industries.
The Dairy Connection
When it comes to calcium peroxide, it's important to delve deeper into its potential interaction with dairy products and its relevance for individuals who follow a dairy-free diet. While calcium peroxide itself is not derived from dairy sources, understanding the relationship between this compound and dairy can provide valuable insights.
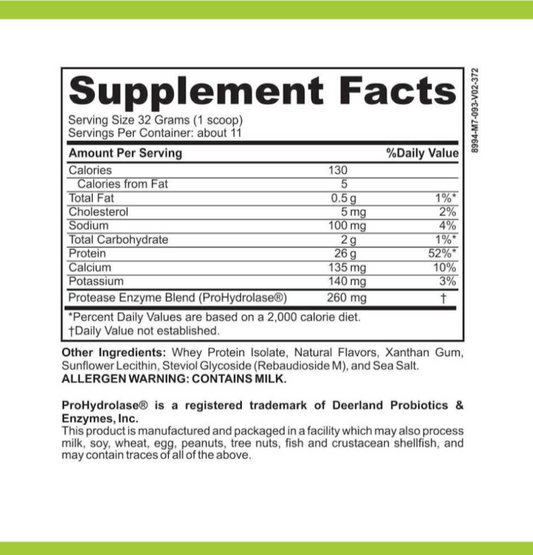
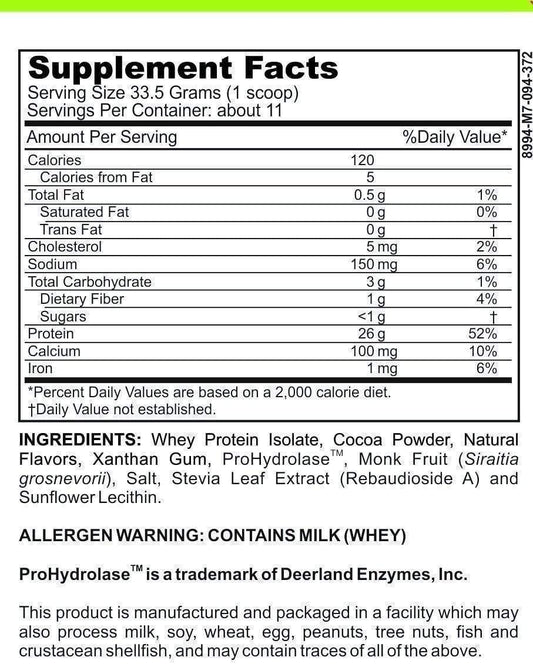
Let's first explore the world of common dairy allergens. Dairy allergies occur when the immune system reacts hypersensitively to proteins found in milk and other dairy products. Among the most prevalent dairy allergens are casein and whey, both of which are animal-derived proteins. For individuals with dairy allergies, strictly avoiding these allergens is crucial to prevent adverse reactions.
Now, let's shift our focus to the role of calcium in dairy products. Calcium is an essential mineral that is found in abundance in dairy products. It plays a vital role in supporting bone health, helping to maintain strong and healthy bones. Additionally, calcium contributes to numerous physiological functions within the body, such as muscle contraction, nerve transmission, and blood clotting.
However, for individuals who follow a dairy-free diet, finding alternative sources of calcium becomes necessary to meet their daily requirements. Luckily, there are various non-dairy sources of calcium available, such as leafy green vegetables (e.g., kale, broccoli), fortified plant-based milk (e.g., almond milk, soy milk), tofu, and certain types of fish (e.g., salmon, sardines).
It's worth noting that while calcium peroxide itself may not have a direct connection to dairy, understanding the broader context of dairy allergies and the role of calcium in dairy products can help individuals make informed choices about their dietary preferences and calcium intake.
In conclusion, the dairy connection goes beyond the mere presence of dairy-derived ingredients in calcium peroxide. It encompasses the understanding of common dairy allergens and the importance of calcium in dairy products. By expanding our knowledge in these areas, we can better navigate dietary choices and ensure adequate calcium intake, even for those who follow a dairy-free lifestyle.
Calcium Peroxide in Food Industry
In the food industry, calcium peroxide can be used as an additive in various food products. This usage extends beyond dairy-based goods, making it relevant for a broader consumer base. Understanding food additives and their sources, as well as the safety and regulations surrounding calcium peroxide use, is essential when considering its presence in dairy-free alternatives.
Calcium peroxide, a compound with the chemical formula CaO2, is a white or yellowish powder that is commonly used in the food industry as an oxidizing agent. It is known for its ability to release oxygen when it comes into contact with water or acidic substances. This property makes it useful in various food applications, such as improving dough quality, enhancing the texture of baked goods, and extending the shelf life of certain food products.
When it comes to food additives, it is important to understand their sources. Food additives can originate from various sources, including natural sources like plants, as well as synthetic sources. In the case of calcium peroxide, it can be synthesized in a laboratory setting or derived from natural sources, depending on the specific manufacturing process.
Food Additives and Their Sources
Food additives are substances that are added to food to improve or preserve its quality. They can enhance the taste, appearance, texture, and nutritional value of food products. These additives can be derived from both natural and synthetic sources.
Natural food additives are substances that occur naturally in plants, animals, or minerals. They can be extracted from these sources and used in food production. For example, natural colorants like beetroot extract or turmeric can be used to add vibrant hues to food products. Natural preservatives like vinegar or salt can help extend the shelf life of certain foods.
Synthetic food additives, on the other hand, are substances that are chemically synthesized in a laboratory. These additives are designed to mimic the properties of natural additives or provide specific functionalities that are not readily available in nature. For instance, synthetic antioxidants like butylated hydroxytoluene (BHT) can be used to prevent the oxidation of fats and oils in food, thereby extending their shelf life.
Calcium peroxide, as an example, can be synthesized or derived from natural sources, depending on the specific manufacturing process. The choice of source can depend on factors such as cost, availability, and desired properties of the additive.
Safety and Regulations of Calcium Peroxide Use
The use of calcium peroxide in food is regulated by authorities to ensure consumer safety. Regulatory bodies, such as the Food and Drug Administration (FDA) in the United States, set guidelines and permissible usage levels for food additives, including calcium peroxide.
It is important to note that the permissible usage levels and specific application guidelines may vary across different countries and regions. These variations can be attributed to differences in risk assessments, cultural preferences, and scientific evaluations conducted by respective regulatory bodies.
Adherence to these regulations is crucial to ensure that food products meet safety standards. Manufacturers and food processors must carefully follow the guidelines provided by regulatory authorities to ensure the safe and appropriate use of calcium peroxide in food production.
Furthermore, thorough testing and evaluation of calcium peroxide and other food additives are conducted to determine their safety for consumption. Toxicological studies assess the potential health risks associated with the use of these additives, considering factors such as exposure levels, potential allergic reactions, and long-term effects.
Overall, the safety and regulations surrounding calcium peroxide use in the food industry aim to protect consumers and ensure that food products are safe, wholesome, and of high quality.
Dairy-Free Alternatives
For individuals who follow a dairy-free diet, finding suitable alternatives to meet their calcium requirements is a priority. This involves exploring plant-based sources of calcium, as well as identifying dairy-free products that may contain calcium peroxide.
Plant-Based Sources of Calcium
Plant-based foods offer a variety of sources for calcium. Dark leafy greens like kale and spinach, as well as tofu and fortified plant-based milk, are excellent sources of dairy-free calcium. Combining these foods in a balanced diet can help individuals meet their nutritional needs.
Dairy-Free Products with Calcium Peroxide
When it comes to dairy-free products that may contain calcium peroxide, it is crucial to read product labels and familiarize oneself with ingredient lists. While calcium peroxide can be used in various food products, its presence is not limited to dairy alternatives. Checking for specific mentions of calcium peroxide in ingredient lists can help identify its presence.
Health Implications
The use of calcium peroxide in food products raises questions about its potential effects on the body. It is important to understand the impacts of calcium peroxide consumption and to consider dietary considerations for dairy-free individuals.
Effects of Calcium Peroxide on the Body
Research on the specific effects of calcium peroxide on the human body is limited. However, it is generally regarded as safe for consumption within the specified limits set by regulatory authorities. As with any food additive, moderation and adherence to recommended usage levels are key to maintaining overall health.
Dietary Considerations for Dairy-Free Individuals
Individuals following a dairy-free diet must pay attention to their overall nutritional intake. While calcium peroxide may contribute to the calcium content of certain dairy-free products, it is important to ensure a well-rounded diet that meets all nutritional needs. Consulting with a healthcare professional or registered dietitian is advisable for personalized dietary guidance based on individual requirements.
Conclusion
In conclusion, calcium peroxide is a versatile compound used in various industries, including the food industry. While calcium peroxide itself is not derived from dairy sources, its usage in food products raises questions about its compatibility with a dairy-free diet. By understanding its chemical composition, common uses, and regulatory guidelines, individuals can make informed choices about calcium peroxide and its presence in dairy-free alternatives. Moreover, individuals following a dairy-free diet can explore plant-based sources of calcium and dairy-free products that offer suitable alternatives to meet their nutritional needs. As with any dietary consideration, consulting with healthcare professionals is advisable for personalized guidance.