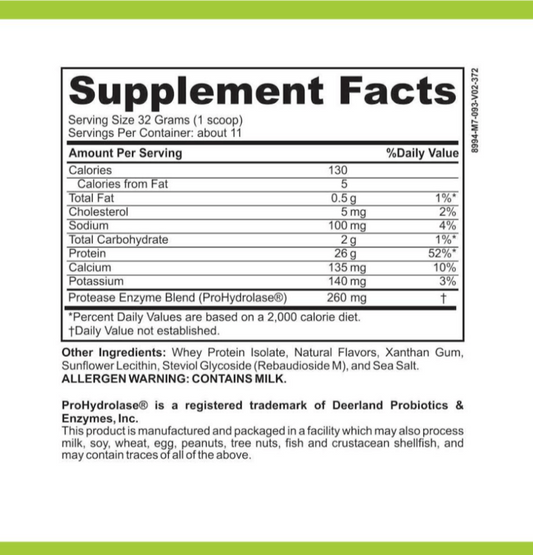
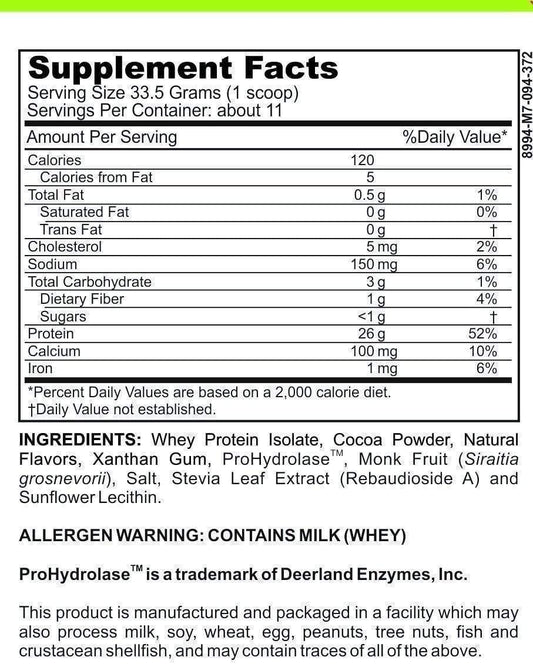
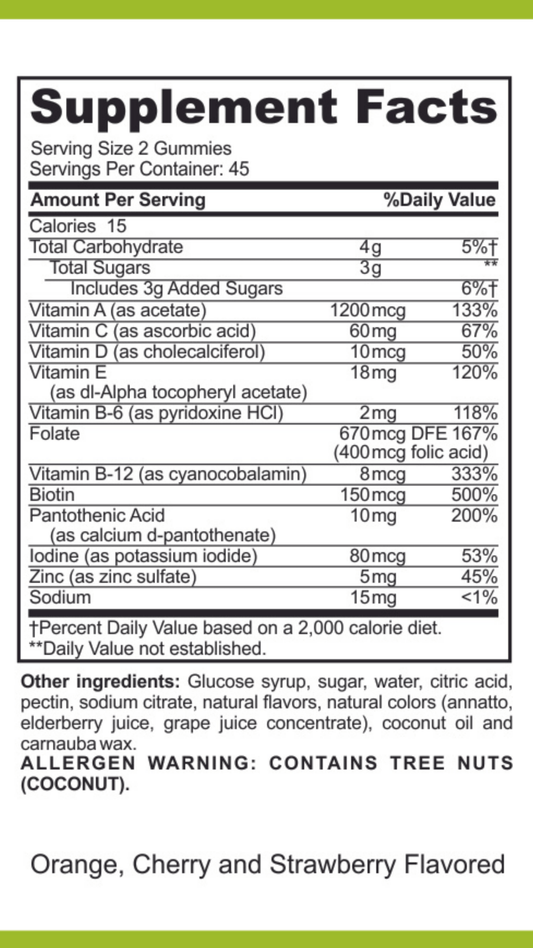
Understanding Enzyme Breakdown: How These Biological Catalysts Function and Degrade
Understanding Enzyme Breakdown: How These Biological Catalysts Function and Degrade
Enzymes are remarkable biological molecules that serve as catalysts for virtually all chemical reactions in living organisms. From digesting your breakfast to replicating DNA, these protein powerhouses make life possible by dramatically accelerating reactions that would otherwise occur too slowly to sustain life. But enzymes themselves aren't immortal—they eventually break down, degrade, and get recycled. Understanding how enzymes function and ultimately degrade provides fascinating insights into the delicate balance of biological systems.
The Fundamental Nature of Enzymes
At their core, enzymes are specialized proteins with a unique three-dimensional structure that allows them to facilitate specific chemical reactions. Unlike many chemical catalysts, enzymes operate with incredible efficiency and specificity. A single enzyme molecule can process thousands of substrate molecules per second, all while working in the relatively mild conditions found inside living cells.
The magic of enzymes lies in their ability to lower the activation energy required for chemical reactions without being consumed in the process. They achieve this by providing an alternative reaction pathway that requires less energy, allowing biological reactions to proceed at rates compatible with life.
The Lock and Key Model
The traditional explanation for enzyme specificity is the "lock and key" model proposed by Emil Fischer in 1894. This model suggests that enzymes have a specific three-dimensional shape that perfectly complements their substrate—like a key fitting into a lock. While this model explains basic enzyme specificity, modern understanding has evolved to recognize that enzymes are more dynamic than originally thought.
Today, the "induced fit" model, proposed by Daniel Koshland in 1958, more accurately describes enzyme-substrate interactions. This model suggests that the enzyme changes shape slightly upon substrate binding, creating an even more ideal environment for the reaction to occur. This flexibility allows enzymes to catalyze reactions with remarkable precision while accommodating slight variations in substrate structure.
The Active Site: Where the Magic Happens
The business end of an enzyme is its active site—a specialized pocket or cleft where substrates bind and chemical reactions occur. Active sites typically comprise just a small portion of the enzyme's total structure but contain precisely positioned amino acid residues that participate directly in the catalytic process.
These active sites create microenvironments that can dramatically alter the chemical properties of bound substrates. They might adjust pH, exclude water, stabilize transition states, or position reactive groups in perfect alignment—all strategies that contribute to their catalytic prowess.
How Enzymes Accelerate Reactions
The catalytic efficiency of enzymes is truly astounding. Reactions that might take years to occur spontaneously can happen in milliseconds with the right enzyme present. This rate enhancement can reach factors of 10^17 or more—a level of acceleration that's difficult to comprehend.
Enzymes achieve this remarkable feat through several mechanisms working in concert. By bringing substrates together in the correct orientation, stabilizing transition states, and providing alternative reaction pathways, enzymes overcome the energy barriers that would otherwise make biochemical reactions prohibitively slow.
Proximity and Orientation Effects
One of the simplest ways enzymes accelerate reactions is by bringing reactant molecules together in the correct orientation. In solution, molecules tumble randomly, and only a tiny fraction of collisions occur with the correct geometry for a reaction. Enzymes solve this problem by binding substrates in precise positions, dramatically increasing the probability of productive interactions.
This positioning effect can increase reaction rates by factors of 1,000 or more, simply by overcoming the entropy barrier that keeps reactants apart or improperly aligned in solution. It's like having a molecular matchmaker that ensures the reactive groups meet in exactly the right way.
Transition State Stabilization
Perhaps the most powerful catalytic strategy employed by enzymes is their ability to stabilize transition states—the high-energy intermediate structures that form during chemical reactions. By preferentially binding to and stabilizing these transition states, enzymes lower the energy barrier that must be overcome for the reaction to proceed.
This principle explains why even small structural changes to an enzyme's active site can dramatically affect its activity. The precise arrangement of amino acids that creates the perfect environment for transition state stabilization is the result of millions of years of evolutionary fine-tuning.
Acid-Base Catalysis
Many enzymes use strategically positioned acidic and basic amino acid residues to donate or accept protons during reactions. These acid-base catalysts can activate substrates, stabilize charged intermediates, and facilitate bond breaking and formation—all contributing to the enzyme's catalytic efficiency.
What makes enzymatic acid-base catalysis particularly effective is that these catalytic groups operate in a precisely controlled microenvironment, allowing them to have unusual pKa values and reactivity compared to the same groups in solution. This environmental tuning is another example of the sophisticated molecular engineering that makes enzymes such powerful catalysts.
Factors Affecting Enzyme Activity
Despite their remarkable efficiency, enzymes are sensitive to their environment. Various factors can enhance, inhibit, or completely deactivate enzymatic activity. Understanding these factors is crucial for both appreciating how enzymes function in living systems and for practical applications in medicine, industry, and research.
Temperature Effects
Like most chemical reactions, enzyme-catalyzed reactions generally proceed faster at higher temperatures—up to a point. As temperature increases, molecules move more rapidly, increasing the frequency of collisions between enzymes and substrates. However, proteins have a critical temperature threshold beyond which their three-dimensional structure begins to unravel, a process called denaturation.
This creates the characteristic bell-shaped curve of enzyme activity versus temperature. Each enzyme has an optimal temperature range where activity peaks before declining as thermal denaturation takes over. For human enzymes, this optimum typically hovers around body temperature (37°C), while enzymes from thermophilic organisms that live in hot springs can remain stable at temperatures exceeding 80°C.
pH Dependence
The pH of the environment dramatically affects enzyme activity by altering the ionization state of amino acid residues in both the enzyme and substrate. Each enzyme has an optimal pH range where its catalytic activity reaches maximum efficiency. Outside this range, changes in protein charge can distort the active site, weaken substrate binding, or directly interfere with the catalytic mechanism.
This pH sensitivity explains why different digestive enzymes work best in different sections of the digestive tract. Pepsin functions optimally in the highly acidic environment of the stomach (pH 2), while pancreatic enzymes work best in the slightly alkaline conditions of the small intestine (pH 8).
The Inevitable Breakdown: How Enzymes Degrade
Despite their remarkable capabilities, enzymes don't last forever. Like all proteins, they eventually succumb to various degradation processes. Understanding enzyme degradation is crucial for appreciating the dynamic nature of cellular metabolism and has important implications for enzyme-based therapeutics and industrial applications.
Thermal Denaturation
Heat is perhaps the most straightforward cause of enzyme degradation. As temperature increases, the kinetic energy of atoms within the protein also increases, eventually overcoming the relatively weak non-covalent forces (hydrogen bonds, ionic interactions, and hydrophobic effects) that maintain the enzyme's three-dimensional structure.
When thermal denaturation occurs, the precisely folded structure of the enzyme unravels, destroying the specific geometry of the active site. While some enzymes can refold correctly if the temperature decreases quickly enough, prolonged exposure to high temperatures typically results in irreversible denaturation and aggregation of the unfolded protein chains.
Chemical Modifications
Enzymes are vulnerable to various chemical modifications that can impair their function. Oxidation, particularly of methionine and cysteine residues, is a common form of enzyme damage. Reactive oxygen species generated during normal cellular metabolism or environmental stress can attack these susceptible amino acids, altering the enzyme's structure and function.
Other chemical modifications include glycation (the non-enzymatic addition of sugar molecules to proteins), deamidation of asparagine and glutamine residues, and various covalent modifications that accumulate over time. These changes gradually alter the enzyme's properties, often reducing its catalytic efficiency or stability.
Proteolytic Degradation
In living systems, enzymes are continuously synthesized and degraded as part of normal protein turnover. Specialized proteolytic enzymes and complexes, such as the ubiquitin-proteasome system, recognize and degrade damaged or unnecessary enzymes. This controlled degradation is essential for maintaining cellular homeostasis and responding to changing metabolic needs.
The rate of enzyme turnover varies widely—some enzymes have half-lives of minutes, while others persist for days or weeks. This variation reflects the different functional roles and regulatory requirements of different enzymes within the complex network of cellular metabolism.
Extending Enzyme Lifespan: Strategies and Applications
Given the importance of enzymes in countless biological and industrial processes, considerable effort has gone into developing strategies to extend enzyme lifespan and stability. These approaches range from evolutionary adaptations in nature to sophisticated engineering techniques in the laboratory.
Understanding how enzymes can be stabilized has profound implications for enzyme-based therapeutics, industrial biocatalysis, biosensors, and numerous other applications where enzyme longevity is desirable.
Natural Stabilization Mechanisms
Nature has evolved various strategies to stabilize enzymes in challenging environments. Extremophile organisms—those living in extreme conditions of temperature, pH, salinity, or pressure—produce enzymes with remarkable stability. These natural solutions include increased numbers of salt bridges and hydrogen bonds, more rigid structures, and specialized adaptations to particular environmental challenges.
Studying these natural adaptations provides valuable insights for protein engineers seeking to enhance enzyme stability for biotechnological applications. Techniques like directed evolution can mimic natural selection in the laboratory, producing enzymes with improved stability while maintaining or enhancing catalytic activity.
As our understanding of enzyme structure, function, and degradation continues to deepen, we gain not only fundamental insights into the chemistry of life but also practical tools for addressing challenges in medicine, industry, and environmental sustainability. These remarkable biological catalysts, with their elegant complexity and astonishing efficiency, remain at the frontier of scientific discovery and technological innovation.